Download PDF
Which treatment is best for vitreous hemorrhages due to proliferative diabetic retinopathy (PDR)? At 24 weeks, intravitreal aflibercept (Eylea) equaled vitrectomy plus panretinal photocoagulation (PRP) in effectiveness, a study from the DRCR Retina Network found.1
“Vitreous surgery has been the standard treatment for vitreous hemorrhages in patients with PDR since the 1970s. But there was evidence from published small studies showing that intravitreal anti-VEGF therapy was effective in stimulating regression of neovascularization and allowing vitreous blood to clear,” thus potentially reducing the need for surgery, said Andrew N. Antoszyk, MD, protocol chair for the study. “We designed a trial to determine whether medical therapy with intravitreal aflibercept could be a viable first-line alternative to surgery in these eyes.”
The answer: Yes, it is. “Furthermore, neither surgical nor medical treatment had a persistent visual advantage over the other during the two years of follow-up,” said Dr. Antoszyk, who practices in Charlotte, North Carolina.
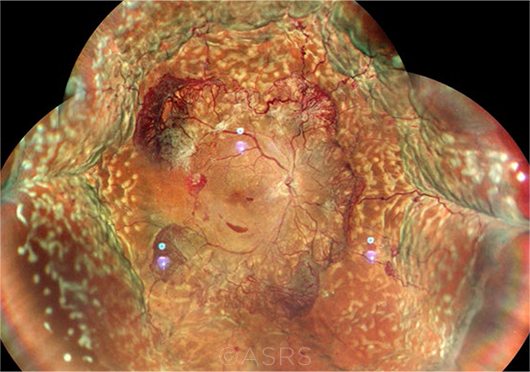 |
ANTI-VEGF CANDIDATE? One week following PRP, this patient with PDR presented with 360-degree choroidal effusion. This image was originally published in the ASRS Retina Image Bank. Manish Nagpal, MD, FRCS, and Sham Talati. Proliferative Diabetic Retinopathy with Choroidal Effusion Status Post PRP. Retina Image Bank. 2020; Image Number 68406. © The American Society of Retina Specialists.
|
Study protocol. At 39 sites in the United States and Canada, the researchers randomized 205 patients with monocular, diabetes-related vitreous hemorrhages to first-line treatment with either 2-mg injections of aflibercept (n = 100) or vitrectomy/PRP. The primary outcome was mean visual acuity (VA) 24 weeks after the initial procedure, with secondary measurements at the one- and two-year marks. Patients could receive the other modality as necessary during the study period.
Outcomes. Four weeks after initial treatment, the mean VA letter score was 52.6 (20/100 Snellen equivalent) in the aflibercept group, versus 62.3 (20/63 Snellen equivalent) in the vitrectomy group (p = 0.003). This was an adjusted mean difference of 11.2 letters favoring vitrectomy. However, at 24 weeks, the mean VA letter scores were no longer significantly different, with outcomes of 69.4 letters in the aflibercept eyes, compared to 69.0 in the vitrectomy group (20/40 Snellen for both; p = 0.88).
At two years, mean VA remained similar between the two groups but lacked statistical significance: 73.7 letters in aflibercept recipients, versus 71.0 in the vitrectomy group (20/40 Snellen for both; p = 0.36).
Number of injections. Patients initially treated with aflibercept required a mean of 8.9 intravitreal injections over two years, and a third of them eventually had to undergo vitrectomy with PRP, Dr. Antoszyk said. “Conversely, a third of the primary vitrectomy group later was treated with aflibercept” and needed only a mean of 2.3 injections, he noted.
Nuances to consider. Improvement in mean VA occurred faster in the primary vitrectomy patients, Dr. Antoszyk said. “At four weeks, there was a 2-line acuity difference [between the two treatments], favoring vitrectomy. That trend began to dissipate as you approached 12 weeks—and this continued until, at 24 weeks, there was just a 0.5 letter difference.”
This pattern suggests that treatment for diabetes-related vitreous hemorrhages can safely be guided by each patient’s visual needs and preference, without sacrificing longer-term VA, Dr. Antoszyk said. For instance, a patient with poor vision in the contralateral eye might prefer vitrectomy/PRP as primary therapy, in order to regain vision in the treated eye quickly, he said. Conversely, patients averse to undergoing surgery can be treated initially with aflibercept, with the goal of either avoiding surgery or postponing it for as long as possible.
The study also showed that less dense hemorrhages are the likeliest to clear quickly with aflibercept, Dr. Antoszyk said. “If the affected eye has a VA of better than 20/800, that’s a subpopulation that recovers more quickly because their hemorrhage is not as dense. They may be able to benefit from intravitreal injections as their primary therapy.”
The findings from the trial are reassuring in a time of potential pandemic-related delays in surgeries, he said. “If patients can’t get to the OR, we now know that aflibercept is a great alternative. You would at least be buying them time to stabilize the neovascularization and allow the blood to clear sufficiently,” Dr. Antoszyk said. And if their hemorrhages recur, he said, “you know that over this time course, even if you had to delay surgery, you’d still have an excellent visual outcome.”
—Linda Roach
___________________________
1 Antoszyk AN et al. JAMA. 2020;324(23):2383-2395.
___________________________
Relevant financial disclosures—Dr. Antoszyk: Jaeb Center for Health Research: C; Genentech: C,S; Regeneron: C; Roche: C,S.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Antoszyk Genentech: C,S; Jaeb Center for Health Research: C; Novartis: C; Opthea: C; Regeneron: C; Roche: C,S.
Dr. Fekrat None.
Dr. Grewal None.
Dr. Nath None.
Dr. Yiu Alimera: C; Allergan: C; Carl Zeiss: C; Clearside Biomedical: C,S; Genentech: C,S; Iridex: C,S; Intergalactic Therapeutics: C; Topcon: C; Verily: C.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review