By Linda Roach, Contributing Writer, interviewing Alison G. Abraham, MHS, MS, PhD; Carol Y. Cheung, PhD; Sharon Fekrat, MD; Dilraj S. Grewal, MD; and Tien Y. Wong, MD, PhD
Download PDF
Modern ophthalmic imaging techniques are uncovering multiple lines of evidence that structural and functional changes in the retinal microvasculature could be the surrogate biomarkers needed for physicians to detect Alzheimer disease (AD) noninvasively at its earliest—and, potentially, most treatable—stages.
Although the specific reasons for correlations between retinal signs and the characteristic neurodegeneration in AD remain murky, ophthalmic researchers express confidence that the many investigations being conducted by large collaborative groups around the world will lead to clinically useful discoveries.
Crucially, these groups are assembling vast libraries of retinal images acquired with specialized techniques, such as optical coherence tomography angiography (OCTA). They also plan to develop artificial intelligence (AI) algorithms to find patterns in the images that the human eye can’t detect.
A Window to the Brain
“There is evidence of correlations between the health of the eye and [that] of the brain because the two are directly connected. And the retina exists as an extension of the central nervous system, offering a window to study brain changes—particularly the blood vessel changes and the levels of neurodegenerative damage in the brain,” said Carol Y. Cheung, PhD, at the Chinese University of Hong Kong.
Alison G. Abraham, MHS, MS, PhD, at Johns Hopkins University in Baltimore, agreed. “It’s not hard to believe that there would be a link between what’s happening in the retina and in the brain, because the eye is an offshoot embryologically of the brain. So the fact that we see parallels should not be surprising.” She added, “The eye shares a lot of common features with the brain—the microvasculature is regulated in a similar way, and both are patterned similarly.”
Tracking changes. “At this point, a good number of imaging studies are showing that there are differences that we can see in the eye that are related to Alzheimer. Those seem to be both microvascular and neuronal,” Dr. Abraham said. For instance, she pointed out, “Studies have found differences in one part or another of the nerve fiber layer in patients with already-diagnosed AD, or they found differences in various aspects of the perfusion of the microvasculature. That has established the relationship.”
However, distinctive retinal differences that inform our understanding of preclinical disease processes have yet to be found, Dr. Abraham noted. “The big question we’re trying to answer is: Can we identify some period during the long-term progression of cognitive disease, when we might be able to see things in the eye that would allow us to intervene effectively?”
Potential benefits for drug trials. This knowledge might hasten the development of therapies for AD, because biomarkers for early-stage dementia, called mild cognitive impairment (MCI), would enable drug companies to determine the impact of potential new therapies over time in people with preclinical disease, before profound and irreversible brain damage has occurred.
“Hundreds of clinical trials looking at novel therapeutics for Alzheimer have been unsuccessful,” said Sharon Fekrat, MD, at Duke University in Durham, North Carolina. Earlier this year, for instance, Biogen and Roche halted AD trials because of lack of efficacy. “We don’t really know why this happened, but one potential reason could be that the individuals entered into these studies had advanced disease, too advanced for the medication being studied to show benefit,” Dr. Fekrat said.
 |
|
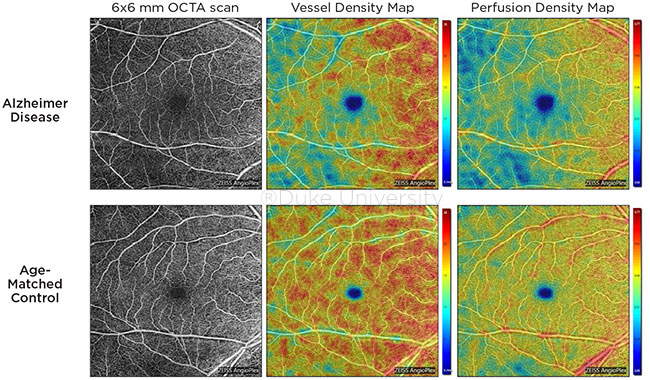
COMPARISON. OCTA images of the superficial capillary plexus of the right eye from a patient with AD (top row) and an age-matched control (bottom row). Corresponding quantitative color maps of vessel density (middle column) and perfusion density (right column) with their respective color scales on the right show decreased vessel density and perfusion density in the subject with AD compared to the control.
|
Potential Biomarkers
Dr. Fekrat and Dilraj S. Grewal, MD, also at Duke, recently reported on OCT and OCTA findings in the superficial capillary plexus in eyes of patients with MCI and AD. They found reduced macular vessel density, perfusion density, and ganglion cell-inner plexiform layer thickness in eyes with AD, compared with MCI and normal controls.1
Other possible retinal biomarkers for early Alzheimer that are under exploration include the following:
- Ultra-widefield, nonmydriatic scanning laser ophthalmoscopy to acquire images showing the presence and status in the retinal periphery of hard drusen, which may be associated with early AD.2
- Near-infrared fluorescence ocular imaging and other tests to look for beta-amyloid protein in the retinas of eyes with preclinical AD.3
- Structural OCT scanning to quantify volumetric alterations in certain areas of the retina.4
- Differences in microcapillary tortuosity and in “retinal fractal dimensions” (vascular branching complexity).5
- The influence of comorbidities such as diabetes, hypertension, and glaucoma on retinal signs associated with AD.
- The status of capillaries deeper inside the retina, in the deep capillary plexus and the whole capillary plexus, compared to the smaller microvessels in the superficial plexus. (Dr. Fekrat’s group is investigating this angle.)
 |
|
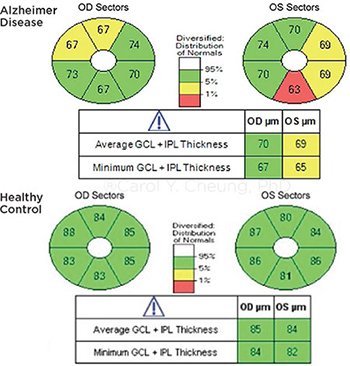
COMPARISON. Ganglion cell inner plexiform layer thinning in a patient with AD (top), compared with a cognitively normal subject (bottom).
|
Image Repositories
Large numbers of retinal images are necessary to provide the raw data needed to analyze imaging data using AI techniques, said Tien Y. Wong, MD, PhD, at the National University of Singapore. “We need better international collaborative framework to share images and validate algorithms.”
Dr. Wong led the multidisciplinary, multinational team that reported the validation of a deep learning algorithm capable of automatically detecting referable diabetic retinopathy—a project that required nearly 500,000 fundus photographs.6
Sample datasets needed. “Our recent success in AI/deep learning on diabetic retinopathy is being transferred to other systemic diseases, including dementia,” Dr. Wong said. “However, we remain limited by good quality and large sample datasets on AD.”
To address this deficit, the Duke research group is collecting multimodal retinal images in eyes of persons with a variety of neurodegenerative diseases and placing them into an open-access repository of deidentified images that they and researchers elsewhere can analyze. In addition to angiograms from controls and AD/MCI patients, the repository recently began adding images from patients with other neurodegenerative diseases, including Parkinson disease, amyotrophic lateral sclerosis, and Huntington disease, Dr. Grewal said.
“We would want to collect images from all over the world into a central registry that all researchers can access,” Dr. Grewal said. “We imaged 500 patients in the last 10 months; we’re moving quickly. We are looking to get images from several thousand or more patients, at least.”
Similarly, Dr. Abraham and her colleagues at Johns Hopkins are gathering OCTA and structural OCT scans from about 1,200 people with normal cognitive ability for a longitudinal four-year study that will monitor subjects’ retinal microvasculatures and cognitive abilities. This study is known as EyeDOC (for “Eye Determinants of Cognition”), Dr. Abraham said.
Call for Collaboration
The image repositories will facilitate multidisciplinary and multi-institutional collaborations in the hunt for retinal signposts of AD, Dr. Fekrat said. “It’s very early, and I think the more people involved in this, the better. To have an impact in our lifetimes we need collaboration. We can’t be in our silos to try to figure this out.”
Her group at Duke includes ophthalmologists, a neurologist, radiologists, and computer engineers with AI expertise. The Duke team is collaborating with international teams who employ ultra-widefield scanning laser ophthalmoscopy to image peripheral hard drusen and other fundus vascular biomarkers.2 And in Singapore, Dr. Wong has coauthored papers about retinal diseases and cognition with scientists from around the world.
Will multimodalities be key? Ultimately, it is unlikely that any single test of retinal structure or function will be enough to screen patients for early AD and/or track its progress in individuals, Dr. Cheung said. “I think we will want to be looking at the whole retina using different modalities, a whole suite of biomarkers.”
Dr. Grewal agreed. “We will likely need multimodal retinal and optic nerve images to optimize the accuracy of an MCI or AD diagnosis. You will want to capture as much information as you can to improve the sensitivity and specificity of the biomarker suite, which may allow us to stratify the various stages of disease and monitor response to new treatments in the context of clinical trials.”
Furthermore, even if one high-tech metric, such as OCTA, proved to be the best single method for detecting microcirculatory signs of early AD, “whether it really can be used [outside of a clinical study] is not clear,” Dr. Abraham said. “These are people in their 70s, 80s, and even 90s, and we’re asking them to sit very still and fixate on a blue light. This can be difficult for them. What if the people that we have the hardest times imaging are the ones that have the highest risk?”
Further Reading
Chan VTT et al. Spectral-domain OCT measurements in Alzheimer disease: A systematic review and meta-analysis. Ophthalmology. 2019;126(4):497-510.
Cheung CY et al. Potential retinal biomarkers for dementia: What is new? Curr Opin Neurol. 2019;32(1):82-91.
Cheung CY et al. Microvascular network alterations in the retina of patients with Alzheimer disease. Alzheimers Dement. 2014;10(2):135-142.
Cheung CY et al. Imaging retina to study dementia and stroke. Prog Retin Eye Res. 2017;57:89-107.
Lee MJ et al. Application of optical coherence tomography in the detection and classification of cognitive decline. J Curr Glaucoma Pract. 2018;12(1):10-18.
O’Bryhim BE et al. Association of preclinical Alzheimer disease with optical coherence tomography angiography. JAMA Ophthalmol. 2018;136(11):1242-1248.
Ting DSW et al. Artificial intelligence and deep learning in ophthalmology. Br J Ophthalmol. 2019;103(2):167-175.
|
Reality Check
“Right now, none of this is ready for prime time,” Dr. Fekrat cautioned. “No one can go to their ophthalmologist and say, ‘Please look at my retina and tell me if I am going to get AD.’”
However, she said, “when the details have been sorted through in the coming years, we may potentially be able to use this multimodal imaging approach to diagnose Alzheimer during the 20-year, relatively asymptomatic period of neuropathogenesis, for earlier clinical trial intervention.”
Dr. Fekrat added, “Ultimately, we would hope to gather enough information to convert these multimodal tests into more easily understood numbers, like physicians do now with the lipid panel blood test.” In such a scenario, she said, “If the quantitative number on one test were higher than X, and this other number were less than Y, and so on, then your risk of Alzheimer would be a certain percentage. But we’re a long way from that.”
___________________________
1 Yoon SP et al. Ophthalmol Retina. 2019;3(6):489-499.
2 Csincsik L et al. Ophthalmic Res. 2018;59(4):182-192.
3 Yang J et al. Mol Imaging Biol. 2019;21(1):35-43.
4 Uchida A et al. Invest Ophthalmol Vis Sci. 2018;59(7):2768-2777.
5 Jung NY et al. J Neurol Sci. 2019;396:94-101.
6 Ting DSW et al. JAMA. 2017;318(22):2211-2223.
___________________________
Dr. Abraham is associate professor of ophthalmology, director of the Wilmer Biostats Center, and principal investigator for the EyeDOC study at Johns Hopkins University in Baltimore. Relevant financial disclosures: None.
Dr. Cheung is assistant professor of ophthalmology and visual sciences at the Chinese University of Hong Kong. Relevant financial disclosures: None.
Dr. Fekrat is professor of ophthalmology and associate professor of surgery at Duke University in Durham, N.C. She directs the departmental faculty mentoring program and its Vitreoretinal Surgery Fellowship Program and is associate chief of staff at the VA Medical Center in Durham. Relevant financial disclosures: None.
Dr. Grewal is associate professor of ophthalmology at Duke University Medical Center and director of grading at the Duke Reading Center in Durham, N.C. Relevant financial disclosures: None.
Dr. Wong is professor and medical director of the Singapore National Eye Center, chairman of the Singapore Eye Research Institute, and vice dean of the Duke-NUS Medical School, all at the National University of Singapore. Relevant financial disclosures: EyRiS: O; plano: O.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Abraham Hirslanden Klinik: C; Implementation Group: C.
Dr. Cheung None.
Dr. Fekrat Alcon: P; Regeneron: C.
Dr. Grewal Alimera: C; Allergan: C; Clearside C.
Dr. Wong Allergan: C; Bayer: C; Boehringer-Ingelheim: C; EyRiS: O; Genentech: C; Merck: C; Novartis: C; Oxurion: C; plano: O; Roche: C.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|