By Linda Roach, Contributing Writer, interviewing Sophie J. Bakri, MD, Kristin Carter, MD, Felipe E. Dhawahir-Scala, MBBS, and Michael D. Ober, MD
Download PDF
When a patient has already lost vision to age-related macular degeneration (AMD), the additional visual disability from a cataract might seem inconsequential. But studies have shown that it is not.
“Before really looking at the data it was unclear whether patients with fluid under the retina with wet AMD should undergo cataract surgery,” said Sophie J. Bakri, MD, at the Mayo Clinic in Rochester, Minnesota.
Work with the retina specialist. Assuring the best surgical outcomes in these patients requires good communication between the cataract surgeon and the retina specialist, Dr. Bakri said. “Make sure that the retina surgeon knows that you are recommending cataract surgery,” she said. “If the patient is on an observation phase, the retina specialist may want to monitor more closely, for example.” Additionally, she noted, the retina specialist “may be reluctant to extend the interval between injections during the cataract surgery period.”
What Surgery Offers
Improvements in visual acuity. In 2018,1 Dr. Bakri and her colleagues reported that 72 AMD patients (81 eyes) with stable preoperative fluid did well after cataract surgery, with no worsening of the neovascular process.
With regard to pre- and postoperative best-corrected visual acuity (BCVA), pre-op BCVA was 20/40 or better in six eyes (7.4%), between 20/50 and 20/100 in 44 eyes (54.3%), and worse than 20/100 in 31 eyes (38.3%). Six months postoperatively, that had improved to 20/40 or better in 49 eyes (60.5%), between 20/50 and 20/100 in 25 eyes (30.9%), and worse than 20/100 in only seven eyes (8.6%).
In another retrospective study, Michael D. Ober, MD, and his colleagues at Henry Ford Hospital in Detroit reported improved VA after cataract surgery in patients with wet AMD who were receiving anti-VEGF injection therapy.2 BCVA improved from 20/89 to 20/53 (converted Snellen acuity) in the cataract extraction group after six months of follow-up and was significantly better than in the nonsurgical (control) group (p = 0.049), who also received anti-VEGF injections. The improvement in BCVA in the control group (29/89 to 20/69) was not statistically significant.
“The most important conclusion isthat cataract surgery still can be very beneficial in patients undergoing treatment for wet AMD,” said Dr. Ober, whois also at the Oakland University William Beaumont School of Medicine in Rochester, Michigan.
Other benefits. Even if the expected improvement in VA is minimal, patients with AMD may be looking for other visual benefits, said Kristin Carter, MD, who is in practice in Tucson, Arizona. “They tell me they have trouble with other aspects of their vision—for example, glare when in a car at night. You can do surgery for glare without really expecting improvement in VA,” Dr. Carter said.
“And I have some patients who are artists, who still do their art despite their AMD, and they have a lot of trouble with color perception if they also have a cataract,” she noted.
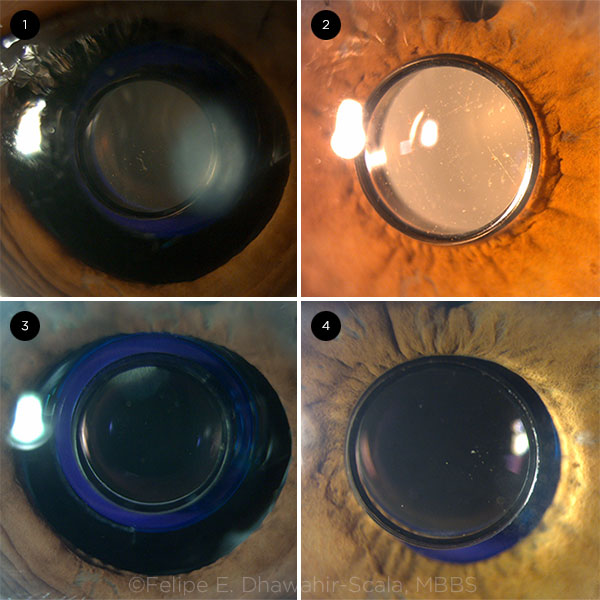 |
|
POTENTIAL IOL. The IMT IOL at (1) one day, (2) two months, (3) four months, and (4) three years postoperatively.
|
A Question of Timing
Stabilize leaky vessels preoperatively. It’s advisable to delay cataract surgery until anti-VEGF injection therapy has reduced any subretinal fluid to as near zero as possible.
As Dr. Ober said, “First and foremost, that patient should not get cataract surgery without being on some sort of a treatment schedule, and you should maximize the treatment before the surgery. Ideally, there should be no subretinal fluid or cystic changes in the retina before you begin to operate.”
However, in some cases a small amount of fluid is still present despite monthly anti-VEGF injections. In those eyes, the preoperative goal would be stabilization, Dr. Bakri said. “With some patients, you just can’t get rid of all the fluid, and there’s no point in delaying cataract surgery for them. As long as they’re being treated and actively monitored, then it’s safe to do the cataract surgery.”
Consider injection schedules. Dr. Carter said that she coordinates the surgery date with the patient’s anti-VEGF treatment schedule.
“Some retina doctors will have me time my cataract surgery two weeks after their last injection. And then I tell my patients they can resume their normal schedule of injections after the surgery,” Dr. Carter said.
Dr. Ober added, “You want them on the maximum schedule of injections around the perioperative period. They can be on a treat-and-extend protocol, but you should reduce the interval around the cataract surgery, and then you can extend afterward.”
Additional Considerations
When OCT is needed. “If patients are not getting injections because they have been fluid-free for a while, or because they only have dry AMD, then they need to have an optical coherence tomography (OCT) scan prior to scheduling the cataract surgery,” Dr. Bakri said. “And if there’s any evidence of fluid on the OCT, they need to be actively treated first.”
When to use NSAIDs. Diabetics are more prone to inflammation after cataract surgery, which can lead to cystoid macular edema, Dr. Bakri said. “So with diabetics, I recommend preoperative topical nonsteroidal anti-inflammatory drugs (NSAIDs) for about two weeks,” and she continues them “for at least a month afterward, because the NSAIDs will target all the other proinflammatory cytokines.”
For the same reason, Dr. Ober said, he has AMD patients use a postoperative topical steroid until their next anti-VEGF injection. In his study of patients who received anti-VEGF injections,2 “We reported a mild increase in retinal thickness and cystic changes following cataract surgery compared to the nonsurgical control group, which is due to either postoperative cystoid macular edema or increased choroidal neovascularization.” As a result, they recommend using anti-inflammatory drops as well as reducing the injection interval in the perioperative period, he said.
Manage patient expectations. The quality of doctor-patient communication is particularly important when a patient has both AMD and a cataract, Dr. Carter said. Some patients may think that their vision will be perfect, and any problems related to their AMD will be resolved following cataract surgery, she said. “Many of their friends without AMD have had [successful] cataract surgery, so they expect the same result.”
Dr. Carter prepares her patients by telling them, “I can take your cataract out, and I think it’s going to help—but because of the AMD, I don’t think it’s going to make your vision perfect, and here’s why.” She emphasized, “From the first time you’re evaluating the patient, it is important to communicate that to them.”
Another challenge involves telling patients with AMD that cataract surgery isn’t appropriate for them, Dr. Carter said. “That’s hard emotionally for patients to hear, because they want to do anything they can to improve their vision.”
Which IOL?
Multifocals? No. Torics? Maybe. In most cases, AMD patients undergoing cataract surgery will receive a conventional monofocal IOL. Multifocal lenses should be avoided, Dr. Ober said. “A multifocal IOL tends to require a fairly pristine macula, so eyes with macular dysfunction do not do well” with these lenses, he said.
If the eye has significant astigmatism (1.5 D or more), a toric IOL might be considered, in order to optimize potential visual acuity, said Felipe E. Dhawahir-Scala, MBBS, at the Manchester Royal Eye Hospital in Manchester, England.
AMD-specific IOLs. Several IOLs designed for eyes with AMD are available internationally. Most outwardly resemble a conventional IOL and are intended to magnify the central visual field to make objects and print easier to see (see “IOLs for AMD”).
“So instead of AMD patients using external magnification devices, with these IOLs they would be using internal devices,” said Dr. Dhawahir-Scala, who published a paper on these implants in 2018.3
The IMT. Only one of the available devices, the Implantable Miniature Telescope (IMT; VisionCare), has extensive published data and is approved for use in the United States. However, the IMT’s steep price tag ($15,000 in the United States), bulky size (it requires a 10- to 11-mm incision), and narrow usage indications have proved to be barriers for surgeons and patients, Dr. Dhawahir-Scala said.
The magnification effect provided by the IMT “is far superior to any of the other devices that are out there, but you need to be very, very, very selective with the patients,” Dr. Dhawahir-Scala said. “Out of 200 patients I have screened in my practice over the last 11 years, I have identified about 40 to 50 patients who were suitable for the implantable telescope, and only three of them had the device implanted, because of the cost.”
The EyeMax Mono. This IOL, from LEH Pharma/Invua Medtech, was launched commercially in Europe in 2017. However, its current status is uncertain following recent action taken by British health officials against its developer.4
In selected research reports published in 2018 and 2019, EyeMax Mono’s optics have been said to generate transverse asphericity, reducing blur in macular areas extending up to 10 degrees from the foveal center.
___________________________
1 Starr MR et al. Am J Ophthalmol. 2018;192:91-97.
2 Saraf SS et al. Am J Ophthalmol. 2015;160(3):487-492.
3 Dunbar HMP, Dhawahir-Scala FE. Ophthalmol Ther. 2018;7(1):33-48.
4 Record of Determinations—Medical Practitioners Tribunal, Sept. 18, 2019. www.mpts-uk.org/-/media/mpts-rod-files/mr-muhammad-qureshi-180919.pdf. Accessed Nov. 19, 2019.
___________________________
Dr. Bakri is a professor of ophthalmology at the Mayo Clinic in Rochester, Minn. Financial disclosures: Alimera: C; Allergan: C; Allegro: C; Carl Zeiss C; EyePoint: C; Genentech: C; Kala: C; Novartis: C; Oxurion: C.
Dr. Carter is a cataract and anterior segment surgeon at Clarity Eye Care and Surgery in Tucson, Ariz. Financial disclosures: None.
Dr. Dhawahir-Scala is a consultant ophthalmologist, vitreoretinal and complex cataracts surgeon, and director of Acute Ophthalmic Services at Royal Manchester Eye Hospital in Manchester, England. Financial disclosures: None.
Dr. Ober is a partner at Retina Consultants of Michigan; associate professor of ophthalmology at the Oakland University William Beaumont School of Medicine in Rochester, Mich.; chief of staff at Straith Hospital for Special Surgery in Southfield, Mich.; and an adjunct teaching faculty member at Henry Ford Hospital in Detroit.
IOLs for AMD
In addition to the IMT and the EyeMax Mono, a handful of other IOLs are being evaluated for eyes with AMD.1
So far, however, the lenses have yet to prove that they can improve the eyesight of patients with wet AMD “in a very substantive way,” Dr. Dhawahir-Scala pointed out.
Lenses under consideration include the following:
IOL-VIP. This device, from Soleko, consists of two separate PMMA lenses that together produce a telescopic effect. One lens goes in the capsular bag while the other is placed in the anterior chamber. A pilot study found that recipients of the first-generation IOL-VIP gained 5 to 7 lines of BCVA. (More recently, Soleko introduced a single-lens version for the capsular bag, the IOL-VIP Revolution.)
Orilens. The foldable Orilens (OptoLight) is an add-on IOL that uses internal mirror optics to magnify objects in the optical center. A randomized controlled clinical trial of Orilens was planned in the United Kingdom, but it was called off for lack of funding.
Scharioth Macula Lens. The SML (Medicontur Medical Engineering) is a bifocal add-on IOL; its central optic area provides high addition power of +10 D. The lens design is intended to improve the near vision of pseudophakes with AMD at a 15 cm working distance.
In a 50-patient prospective trial, the mean corrected near VA improved from 0.23 ± 0.12 preoperatively to 0.57 ± 0.33 at one year, while the mean corrected distance VA remained unchanged.2
Lentis Max. This high-add version of Oculentis’ existing bifocal IOL has a second near segment on the posterior surface that produces 1.5× magnification of objects at 30 cm and 3× magnification at 15 cm.
In 2018, Austrian ophthalmologists reported improved visual functioning after primary implantation of the Lentis Max in a single patient with end-stage AMD and pseudoexfoliation.3
___________________________
1 Dunbar HMP, Dhawahir-Scala FE. Ophthalmol Ther. 2018;7(1):33-48.
2 Srinivasan S. BMJ Open Ophthalmol. 2019;4(1):e000322.
3 Borkenstein AF, Borkenstein EM. Clin Case Rep. 2018;7(1):74-78.
|