By Kyle Huynh, MD, and Neil C. Chungfat, MD
Edited by Steven J. Gedde, MD
Download PDF
Paisley Pangolin* is a 12-year-old girl who began having issues last year with blurry vision, light sensitivity, eye pain, and redness. She saw a local optometrist, who treated her with topical corticosteroids. When her eye problems recurred with cessation of therapy, she was referred to a pediatric ophthalmologist at our institution.
She Arrives at Our Institution
Paisley’s best-corrected visual acuity was 20/20 in the right eye and 20/25 in the left. Her extraocular motility was intact and symmetric, and her visual fields were full on confrontation. There was no afferent pupillary defect or anisocoria. Her intraocular pressure (IOP) was 10 mm Hg in both eyes. The review of systems was negative for nausea, vomiting, malaise, abdominal pain, arthritis, or cutaneous rashes.
At the slit lamp, we noted 1+ diffuse conjunctival hyperemia with fine, stellate keratic precipitates (KPs) in both eyes. The anterior chambers were deep with 1+ cell and flare. Both irides were round and reactive and absent of inflammatory nodules, transillumination defects, or posterior synechiae.
A dilated funduscopic exam was performed, which was unremarkable for anterior vitreous cells or snowballs. The optic nerves were pink and healthy in appearance without edema. The maculas and peripheral retinas were flat and attached without lesions.
Labs include a complete blood count (CBC); a basic metabolic panel (BMP); and tests for angiotensin-converting enzyme (ACE), antinuclear antibody (ANA), human leukocyte antigen (HLA)-B27, rheumatoid factor (RF), rapid plasma reagin (RPR), Treponema pallidum passive particle agglutination (TP-PA), and Lyme disease.
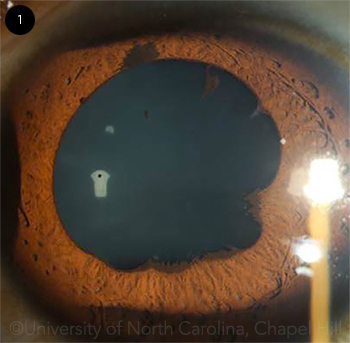 |
|
HER LEFT EYE. We noted temporal posterior synechiae and pigment clumps on the anterior lens capsule.
|
Pinning It Down
Differential diagnosis. The differential for bilateral, nongranulomatous anterior uveitis is broad and encompasses idiopathic, infectious, inflammatory, and neoplastic causes.
In the pediatric population, idiopathic anterior uveitis is the most common presentation and accounts for nearly 60% of the cases diagnosed. Conversely, systemic inflammation with ocular features occurs less frequently. This includes etiologies such as juvenile idiopathic arthritis (JIA), sarcoidosis, and HLA-B27–associated uveitis.1
Cases of uveitis that present with abnormal features—including bilateral involvement, granulomatous KPs, or those that do not respond completely to aggressive therapy with topical corticosteroids—deserve a focused lab workup for systemic disease. It is important to rule out syphilis before initiating high-dose systemic steroids or other systemic immunosuppressive medications.
Lab results. Paisley’s serologic tests were largely unremarkable. The syphilis and Lyme serologies were nonreactive. A diagnosis of JIA seemed unlikely in the setting of normal ANA results and her lack of joint complaints. At this point, she was referred to our cornea and uveitis clinic for nonresolving anterior chamber inflammation. Her medical chart was reviewed and a urine beta-2 microglobulin was obtained. This returned elevated at 493 μg/L (normal: 0-300 μg/L). A urinalysis was also obtained, which was positive for leukocyte esterase, proteinuria, and 1+ squamous epithelial cast cells. A diagnosis of tubulointerstitial nephritis with uveitis (TINU) syndrome was made.
Treatment. Paisley had a protracted recovery course. To suppress the inflammation, she was initially treated with topical prednisolone acetate 4 times a day, which was then increased to 6 times a day. However, the inflammation persisted, possibly because of her poor medication compliance. She subsequently developed posterior synechiae in the left eye (Fig. 1), and her vision worsened to 20/40 in the right eye and 20/100 in the left eye. She was then switched to difluprednate (Durezol) 4 times daily to encourage better medication compliance and a cycloplegic drop to break the synechiae. On subsequent visits, her IOP was elevated in the mid-30s in both eyes, likely from the steroid response. She was then startedon timolol/dorzolamide (Cosopt) drops twice a day. Her pressure normalized, but the anterior chamber inflammation had not resolved. It was unclear at this time whether this inflammation stemmed from nonadherence to the recommended use of corticosteroids or demonstrated the inadequacy of topical corticosteroid monotherapy.
After the diagnosis of TINU syndrome was confirmed and ocular syphilis was ruled out, aggressive topical therapy was exchanged for high-dose oral prednisone 60 mg daily (approximately 1 mg per kg), which successfully quieted the ocular inflammation. Her vision improved to 20/25 in both eyes. To balance the risk for side effects with adequate control of the inflammation, Paisley and her mother were instructed to do a gradual taper. The prednisone would be decreased by 10 mg every 2 weeks with close interval follow-ups.
Unfortunately, out of concern about weight gain, Paisley’s mother decreased the cumulative dose to 20 mg daily after just 1 week. The inflammation recurred and oral prednisone was quickly increased back up to 60 mg a day with strict instructions to taper slowly.
Paisley was then referred to pediatric nephrology for evaluation. Her urinalysis showed characteristic features of acute interstitial nephritis (AIN). In contrast, her creatinine function was normal, and she did not exhibit flank pain during her ocular episodes.
Paisley was also referred to a pediatric rheumatologist in anticipation of requiring a steroid-sparing agent to better control her symptoms.
Discussion
TINU syndrome is defined as a combination of idiopathic AIN and bilateral, anterior nongranulomatous uveitis. Dobrin et al. first described this condition in 1975.2 Although the exact pathophysiology remains unclear, it is postulated to involve both T-cell mediated activity and humoral autoimmunity. Certain HLA phenotypes, such as HLA-DQA1*01:04 and DRB1*14 have been associated with TINU syndrome.3 Medication hypersensitivity reactions to NSAIDs and certain antibiotics have also been implicated.
Demographics. Most patients diagnosed with TINU syndrome are girls with a mean age of 15. Ethnicity, familial inheritance, and geographic clustering do not appear to play a role.3
Ocular symptoms. Symptoms may include redness, pain, light sensitivity, and decreased vision. Findings involve anterior chamber inflammation, flare, KPs, and, rarely, iris nodules. The uveitis is predominately anterior, but it can sometimes involve vitreous cells or multifocal chorioretinal lesions.
Systemic symptoms. AIN can manifest as vague flank pain, malaise, or fever. Obtaining a urinalysis and determining urine beta-2 microglobulin levels can be helpful, but a definitive diagnosis requires a renal biopsy.
Natural history. The natural history varies. Ocular symptoms may precede AIN (21%), occur concurrently with AIN (15%), or follow AIN (65%).4 Our case was interesting in that Paisley only had visual complaints, which could have led to a diagnosis of idiopathic uveitis. Fortunately, a high index of suspicion in a pediatric patient with bilateral, recurrent uveitis prompted us to obtain a urinalysis and to test for beta-2 microglobulin, which led to the correct diagnosis. Ocular complications are rare, but recurrence is especially common.
Treatment. The first-line treatment is topical corticosteroids with a slow taper. Systemic corticosteroids can be used, but they must be balanced with known side effects. If corticosteroids cannot be adequately tapered, a steroid-sparing agent such as methotrexate, mycophenolate mofetil, or an anti-TNF (tumor necrosis factor)-α agent can be used.
In a retrospective case series investigating the outcomes of long-term treatment of TINU syndrome, 22% of patients achieved quiescence with topical corticosteroid monotherapy. Conversely, a majority of patients (78%) required a more aggressive and targeted treatment plan to control the inflammation. Of this group, 29% of patients achieved quiescence with high-dose systemic corticosteroids alone, whereas 71% of patients needed to be started on a steroid-sparing immunosuppressant to quiet the inflammation. Treatment course spanned from 16 to 123 months.5 Conversely, AIN symptoms were mostly self-limiting, which may be why Paisley’s creatinine remained normal during her clinical course.
Take-home points. TINU syndrome is a rare diagnosis that should be considered when the clinician is faced with recurrent, bilateral nongranulomatous anterior uveitis in a pediatric patient. AIN symptoms may not always be present, as our case illustrates. Thus, a high index of suspicion combined with a focused workup should be pursued if a case does not respond to empiric therapy or if presenting signs are atypical. Urine beta-2 microglobulin, if not ordered initially, should be strongly considered if the urinalysis is abnormal. Consultation with a nephrologist is advised when managing patients with TINU syndrome.
In summary, our case underscores the challenges of diagnosing this disease and controlling it with corticosteroids alone. As is often the case, coordinated multispecialty care is recommended.
___________________________
* Patient name is fictitious.
___________________________
1 Tugal-Tutkun I. J Ophthalmic Vis Res. 2011;6(4):259-269.
2 Dobrin RS et al. Am J Med. 1975;59(3):325-333.
3 Purt B et al. Am J Case Rep. 2016;17:869-873.
4 Mandeville JT et al. Surv Ophthalmol. 2001;46(3):195-208.
5 Sobolewska B et al. Ocul Immunol Inflamm. 2016. Published online Dec. 12, 2016.
___________________________
Dr. Huynh is a second-year ophthalmology resident and Dr. Chungfat is assistant professor of cornea, cataract, uveitis, and refractive surgery. The authors would like to acknowledge Katherine Whitfield, MD, who is a pediatric ophthalmologist. All are at the Department of Ophthalmology at the University of North Carolina, Chapel Hill. Relevant financial disclosures: None.