By Cameron Holicki, Jake Sims, Nathaniel Gelinas, DO, Tatyana Sherman, DO, Joseph Holicki, DO, and David Kaufman, DO Edited By: Steven J. Gedde, MD
Download PDF
Janet Jenkins* was a witty and active 73-year-old woman, who regularly participated in sewing and enjoyed keeping up with friends and family. She first presented to her optometrist with the chief complaint of a 1-week history of a new green tint to her vision. She had a past medical history of well-controlled type 2 diabetes mellitus and essential hypertension.
Her optometrist informed her that her ocular examination was within normal limits, and her symptoms were likely due to an acute cerebrovascular accident (CVA). Mrs. Jenkins was instructed to follow up with her primary care physician, who ordered noncontrast computed tomography (CT) of the head. The results showed no acute intracranial abnormality, and she was referred to ophthalmology for further evaluation.
We Get a Look
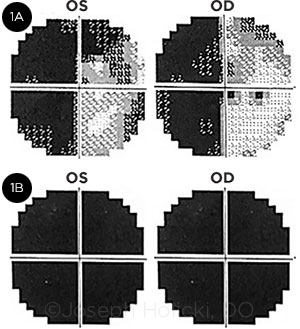
One week later, Mrs. Jenkins presented to the general ophthalmology clinic complaining of new-onset difficulty with writing and worsening vision. Her best-corrected visual acuity at distance was 20/20 in both eyes. Intraocular pressure (IOP) as well as pupil size and reactions were within normal limits. Likewise, the anterior and posterior segment examinations were both within normal limits. However, confrontation visual fields demonstrated a possible left homonymous hemianopia. A subsequent 30-2 Humphrey visual field test confirmed a complete left homonymous hemianopia with an additional peripheral nasal defect in the left eye and a superotemporal defect in the right eye (Fig. 1A).
At that time, the differential diagnosis included acute CVA, atypical brain mass, inflammatory disorders, cerebral vasculitis, and autoimmune encephalopathy. An outpatient magnetic resonance image (MRI) of the brain without contrast, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) levels were ordered. All of the imaging studies and blood work were unremarkable.
 |
|
FIELD LOSSES. Humphrey visual field tests from the patient’s 2 encounters. (1A) Visual fields from the first visit show a complete left homonymous hemianopia with additional superior nasal quadrant defects in the left eye (OS) and a superotemporal defect in the right eye (OD). (1B) Retesting 19 days later shows complete bilateral loss of vision.
|
Follow-up Visit
Mrs. Jenkins returned 19 days later for a follow-up visit. Her husband stated that her personality had changed rapidly since the last appointment, and she had become increasingly confused.
During examination, her speech was dysarthric and dysphonic, with transient perseveration, irregular rhythm, and frequent pauses. She demonstrated dysarthric posturing of her upper extremities. Additionally, she exhibited significant startle myoclonus.
At that time, her visual acuity had decreased to light perception in both eyes. IOP, pupil size and reactions, and the anterior and posterior segment examinations remained within normal limits. A repeat 30-2 Humphrey visual field test showed complete bilateral vision loss (Fig. 1B). However, this study’s reliability was questionable, given the patient’s substantial decrease in visual acuity and mental status changes.
Further evaluation in the ED. Mrs. Jenkins was sent directly to the emergency department for further evaluation. A CT scan of the brain without contrast was unremarkable and stable from her previous study. Lumbar puncture was performed, which showed a normal opening pressure, cell count, protein level, glucose level, and white blood cell count. Cerebrospinal fluid (CSF) was sent for Gram stain, culture, and assessment of numerous infectious and immunological markers, all of which were unrevealing.
A comprehensive meta-bolic panel, blood culture, urine culture, complete blood count, ESR, CRP, urinalysis, thyroid-stimulating hormone with reflex thyroxine (T4), coagulation studies, and an arterial blood gas test were obtained. The findings were all unremarkable.
Differential diagnosis. Given the clinical course and extensive negative workup at that time, the updated differential diagnosis included conversion disorder, rapidly progressive dementia, immune-mediated encephalopathy, prion disease, and leptomeningeal carcinomatosis.
Hospital Stay
Mrs. Jenkins was initially admitted to the psychiatry ward but was transferred to the ICU when an electroencephalogram (EEG) revealed epileptiform discharges arising from the right temporal lobe. Numerous blood, CSF, and imaging studies were performed, none of which revealed a definitive diagnosis. Infectious, immunologic, and neoplastic processes were excluded.
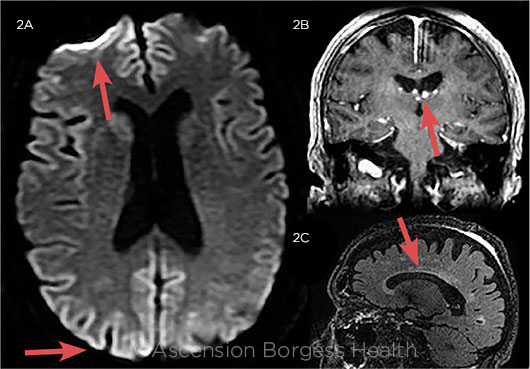
Repeat MRIs with and without contrast, taken 7 days after admission, showed subtle heterogeneity of cortical diffusion on diffusion-weighted imaging (DWI) and fluid-attenuated inversion recovery (FLAIR), most prominent in the bilateral occipital and right frontal cortices (Fig. 2). A repeat EEG, performed the same day, showed independent bilateral left frontal and right frontal 1-Hz periodic lateralized epileptiform discharges with diphasic and triphasic morphology. Neurologically, her mental status continued to decline rapidly.
 |
|
FLAIR FINDINGS. MR images (FLAIR sequence) of Mrs. Jenkins, taken approximately 4 weeks after symptom onset. (2A) Image shows cortical hyperintensities, most prominent in the bilateral frontal lobes and the right occipital cortices. (2B) Enhancing ependymal nodular structures of unclear significance around the lateral ventricles. (2C) Scattered areas of white matter hyperintensity, most notable in the periventricular region.
|
Making the Diagnosis
A repeat CSF analysis tested positive for 14-3-3 and neuron-specific enolase (NSE) protein. These CSF findings, along with the MRI and EEG results, rapidly progressive neurological decline, and prominent startle myoclonus, supported the diagnosis of Creutzfeldt-Jakob disease (CJD). Mrs. Jenkins continued to decline rapidly and passed away 53 days after symptom onset.
Discussion
CJD refers to a group of human transmissible spongiform encephalopathies (also known as prion diseases) that present as rapidly progressive neurodegenerative disorders. The presentation, and course of CJD are highly variable, but the disease is universally fatal.1
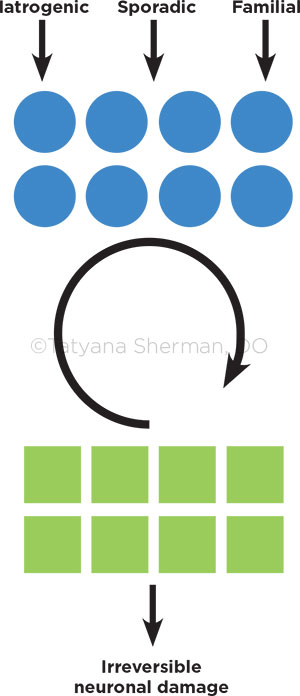
Pathophysiology. The pathophysiology of CJD is not fully understood, but it is related to deposition of misfolded prion proteins in the brain. Natural prion protein (PrPc) is normally found in the synaptic cleft and becomes pathological only when mutated to its stereoisomer (PrPSc). The PrPSc protein is resistant to degeneration by proteases, resulting in accumulation and subsequent degradation of neuronal tissue. Additionally, PrPSc induces native prion protein mutation, creating a feedback loop that is self-sustaining and impossible to control (Fig. 3).2-4
Visual effects. The visual manifestations of CJD are highly variable. They include decreased visual acuity, visual hallucinations, homonymous visual field defects, cortical blindness, micropsia, macropsia, palinopsia, dyschromatopsia, metamorphopsia, and chromatopsia.2-4
A homonymous visual field defect, as in this case, is a common initial presentation of CJD and often leads to a misdiagnosis of an acute CVA. Other common misdiagnoses include primary optic disorders or neurodegenerative disorders such as Alzheimer disease or Lewy body dementia.2,3
Diagnostic considerations. CJD can be definitively diagnosed only by means of brain biopsy with standard neuropathologic techniques. “Probable CJD” can be diagnosed if the patient has rapidly progressive dementia and at least 2 of the following features: myoclonus, visual or cerebellar signs, pyramidal/extrapyramidal signs, and akinetic mutism. In addition, diagnosis requires a positive result on at least 1 of the following studies: atypical EEG (periodic sharp wave complexes) during an illness of any duration; a positive 14-3-3 CSF assay in patients with a disease duration of less than 2 years; or MRI high signal abnormalities in the caudate nucleus and/or putamen on DWI or FLAIR.1
The patient’s husband declined a postmortem brain biopsy; however, her laboratory studies and clinical course met the criteria for “probable CJD.”
Role of protein assay. The diagnostic utility of the CSF 14-3-3 protein assay is controversial, although it has acceptably high sensitivity and specificity within appropriate clinical contexts. A systematic review by Muayquil et al. reported a sensitivity of 92% and a specificity of 80% in diagnosing CJD.5
The utility of the 14-3-3 assay is limited by the pretest probability of CJD, as assessed by patient demographics, clinical course, and results of ancillary testing. Thus, the practitioner’s determination of pretest probability is essential, as the assay will be useful only in patients with a pretest probability of CJD between 20% and 90%.5
CJD subtypes. The mechanism of acquiring the PrPSc mutation can be sporadic, iatrogenic, or familial, with the most common subtype being sporadic.1 Interestingly, iatrogenic CJD has been reported in association with both corneal transplants and tonometry.6 CJD has also been linked to exposure to human brain products, dural grafts, dural electrode implants, and human growth hormone injections.1 Our patient had no history of any such events related to her condition, making sporadic CJD most likely.
Variants. Sporadic CJD can be further divided into 2 subtypes: the Heidenhain and Oppenheimer-Brownell variants.3 The former accounts for only approximately 3.7% to 4.9% of confirmed cases of sporadic CJD.2
The Heidenhain variant is associated with isolated visual symptoms at disease onset and rapid deterioration.2-4 The mean length of time between initial symptoms and death for this variant is 5.7 months, compared to approximately 7.5 months among all patients with definitive sporadic CJD.4 Mrs. Jenkins’ precipitous decline and death 53 days after onset suggest that she had the rare Heidenhain variant.
 |
|
CJD PROTEIN CONVERSION. Schematic illustration of the pathophysiology of Creutzfeldt-Jakob disease. The blue circles represent native prion protein (PrPc), and the green squares represent mutated prion protein (PrPSc). The circular arrow represents the constitutive feedback loop of the conversion of PrPc to PrPSc via the protease resistance of the PrPSc protein.
|
Key Points for Clinicians
Keep CJD in mind. As ophthalmologists, we must keep CJD in the differential diagnosis for various ocular complaints when the initial workup does not reveal a more common etiology.
Recognize the risk of iatrogenic transmission. Moreover, although extremely uncommon, the iatrogenic spread of sporadic CJD via ophthalmic surgeries and examination techniques has been recorded in the literature. Specifically, while definitively confirmed in only a handful of cases, corneal allograft transplantations and tonometry have been linked to CJD cases.6,7
Additionally, there is a theoretical risk of iatrogenic spread of prion proteins during intraocular surgeries. In 2003, Head et al. demonstrated that PrPSc is found in similar concentrations to brain tissue in the neural retina, optic nerve, and retinal pigment epithelium in variant and sporadic CJD cases. Several cases have been reported of misdiagnosed sporadic CJD in patients who underwent ophthalmic surgery shortly after clinical onset. The subsequent use of reusable surgical instruments presented serious potential risk of the spread PrPSc to future patients. Importantly, standard sterilization technique does not adequately eliminate prion proteins from surgical instruments.6,7
The points discussed above highlight the importance—for all practicing ophthalmologists—of knowledge about this rare but devastating disease.
___________________________
*The patient’s name is fictitious.
___________________________
1 Centers for Disease Control and Prevention. Creutzfeldt-Jacob Disease, Classic (CJD). www.cdc.gov/prions/cjd. Accessed March 6, 2018.
2 Baiardi S et al. J Alzheimers Dis. 2015;50(2):465-476.
3 Cornelius JR et al. J Neuroimaging. 2009;19(3):283-287.
4 Wong A et al. J Clin Neurosci. 2015;22(10):1688-1689.
5 Muayqil T et al. Neurology. 2012;79(14):1499-1506.
6 Hamaguchi T et al. Neuropathology. 2009;29(5):625-631.
7 Head MW et al. Invest Ophthalmol Vis Sci. 2003;44(1):342-346.
___________________________
Mr. Holicki and Mr. Sims are third-year medical students at Michigan State University College of Osteopathic Medicine in East Lansing. Dr. Gelinas and Dr. Sherman are PGY1 residents who will begin ophthalmology residency at Ascension St. John Macomb-Oakland Hospital in Detroit in July 2018. Dr. Holicki is a general ophthalmologist at Holicki Eye Center in Coldwater, Michigan. Dr. Kaufman is the founding chair of the Department of Neurology at Sparrow Hospital and a professor and chair of the Department of Ophthalmology and Neurology at Michigan State University in East Lansing, Michigan. Financial disclosures: None.