Download PDF
Glaucoma associated with aphakia and pseudophakia is one of the most common complications of surgery for congenital cataract.1 Although the underlying mechanism remains unclear, it is thought to be multifactorial in origin. Diagnosis relies on clinical signs, such as elevated intraocular pressure (IOP) and optic nerve head cupping, as children are often unable to perform a visual field examination. However, because it is difficult to obtain a good optic nerve examination on a child in the office, the diagnosis can often be missed or confused with ocular hypertension. (For tips on measuring IOP in children, see the Clinical Update, “Do I Really Have to Check That Pressure? Measuring IOP in Children.”)
Furthermore, glaucoma may present years after the cataract surgery; thus, long-term surveillance is essential for accurate diagnosis and timely intervention. This article reviews epidemiology, risk factors, mechanisms, and treatment for glaucoma secondary to pediatric cataract surgery.
Epidemiology
The prevalence of glaucoma after pediatric cataract surgery is reported to be as high as 41%,1 depending on length of follow-up.2 The reported time to diagnosis after surgery averages 6 to 7 years.1,3 For children who have undergone lensectomy, both the diagnosis of secondary glaucoma and delayed glaucoma presentation are associated with a worse visual prognosis.2
Risk Factors
Glaucoma may occur even after an uncomplicated lensectomy for congenital cataract. However, several risk factors have been identified, including surgery in the first year of life, microcornea (corneal diameter less than 10 mm), other ocular abnormalities, certain types of cataract (nuclear sclerotic and persistent hyperplastic primary vitreous), retained lens proteins, and a history of secondary membrane surgery.2,4
Although some investigators have reported a lower rate of glaucoma in eyes implanted with an intraocular lens (IOL), there may be a selection bias in terms of the children chosen for IOL implantation that confounds aphakia as a risk factor.3 The recent 5-year follow-up from the randomized controlled Infant Aphakia Treatment Study (IATS) did not find any significant differences in glaucoma-related adverse events between patients implanted with an IOL and those left aphakic.5
Younger age at the time of cataract surgery is one of the few glaucoma risk factors under a surgeon’s control. However, the current literature is conflicting with regard to the optimal timing for lensectomy. Although delaying surgery might reduce subsequent glaucoma risk, the physician must also take into account the deleterious effect of delays on visual deprivation and amblyopia.3 In unilateral cases, many experts recommend performing congenital cataract surgery at 4 to 6 weeks of age to avoid impaired visual development.6
Although anatomic abnormalities can complicate congenital cataract surgery, modern instrumentation and techniques have decreased rates of previously common unfavorable surgical outcomes, such as retained lens fragments and cortex opacification, as well as the need for secondary surgery for posterior capsule opacification.3
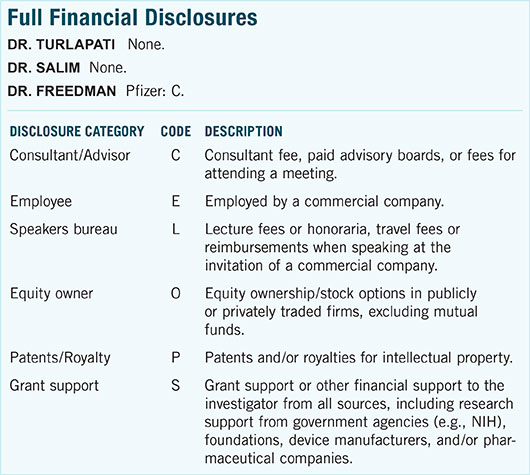
 |
Drainage Implant. Baerveldt device in aphakic eye of young patient with glaucoma secondary to lensectomy. (See Fig. 2 for more details.)
|
Mechanisms
Angle-closure glaucoma. When earlier needling techniques were used to remove congenital cataracts, angle-closure glaucoma was the most common type seen in this setting. Mechanisms included swelling of lens material, fibrinous material blocking the pupil, and vitreous prolapse into the anterior chamber with pupillary block.
Advances in surgical techniques for aspiration, posterior capsulotomy, and anterior vitrectomy have now largely reduced the occurrence of these glaucomas.3
Vajpayee and colleagues have also reported pupillary-block glaucoma after IOL implantation in children.7 Although the exact pathogenesis remains unclear, potential mechanisms include formation of synechiae between the IOL and iris or anterior capsule, vitreous prolapse into the anterior chamber from zonular or posterior capsule compromise, and alterations in the anatomic structures of the drainage angle.
Tactics to reduce the risk of angle-closure glaucoma include postoperative use of steroids and mydriatics and iridectomy.
Open-angle glaucoma. Open-angle glaucoma (OAG) is now the most common type of glaucoma occurring after congenital cataract surgery. The recent IATS reported that 19 of 20 eyes that developed glaucoma had OAG.5 As noted above, it often has delayed onset years after lensectomy.4 Although the exact etiology remains uncertain, mechanical and chemical theories have been postulated.1 Both mechanisms eventually reduce aqueous outflow facility and lead to elevated IOP.
The mechanical etiology points to barotrauma from the surgery and reduced traction on the trabecular meshwork from loose zonular fibers after lens extraction.
The chemical-based theory suggests that contact between the trabecular meshwork and the vitreous, lens particles, or inflammatory cells is likely toxic to the drainage angle.
In addition, steroid medications used in connection with cataract surgery are another cause of IOP increase. Children are more susceptible to steroid-induced ocular hypertension, which often has a rapid onset and is more aggressive than in adults. Because inflammation is increased and of longer duration after pediatric cataract surgery, patients receiving steroids must be monitored closely in the postoperative period.4
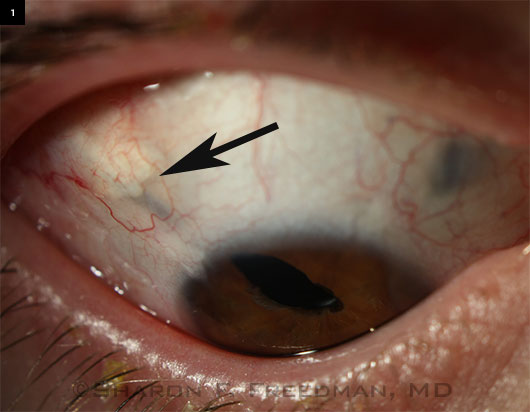
A Complex Case
Right eye of a teenaged boy who was born with microcornea and cataracts and lost his left eye to complications of retinal detachment after glaucoma surgery in infancy. He developed glaucoma in his remaining eye in early childhood.
 Initially managed with glaucoma medications, he later required a drainage implant. A 250-mm Baerveldt device was implanted in the pars plana (in the superotemporal area at about 10 o’clock), and a pars plana vitrectomy was performed to help keep the tube away from the cornea and to avoid vitreous blockage of the tube or traction on the retina. (This procedure was performed jointly with a vitreoretinal surgeon familiar with pediatric cases.) The irregular pupil was present before surgery but worsened afterward, and he required a small second surgery to remove a portion of the iris that was blocking the tip of the tube, which was placed underneath the iris. Initially managed with glaucoma medications, he later required a drainage implant. A 250-mm Baerveldt device was implanted in the pars plana (in the superotemporal area at about 10 o’clock), and a pars plana vitrectomy was performed to help keep the tube away from the cornea and to avoid vitreous blockage of the tube or traction on the retina. (This procedure was performed jointly with a vitreoretinal surgeon familiar with pediatric cases.) The irregular pupil was present before surgery but worsened afterward, and he required a small second surgery to remove a portion of the iris that was blocking the tip of the tube, which was placed underneath the iris.
This patient’s IOP is now controlled with 1 daily glaucoma drop, and he has maintained 20/20 vision for more than 5 years after surgery.
Some patients with congenital cataracts or other ocular abnormalities may have a short axial length. In such cases, alterations in technique may be needed to ensure that the back edge of the drainage reservoir is far enough away from the optic nerve.
—Photographs and case study courtesy of Sharon F. Freedman, MD
|
Medical Treatment
The first-line treatment for glaucoma associated with pediatric aphakia or pseudophakia is usually medication. The response to medical treatment for glaucoma secondary to pediatric cataract surgery is better than that observed with primary congenital glaucoma. A review by Asrani and Wilensky reported that medications alone controlled IOP in 21 of 33 eyes (63.3%).8
Choice of medication. Commonly used drugs include topical beta blockers, carbonic anhydrase inhibitors, and pilocarpine.3 Because of the risk of retinal detachment with pilocarpine, especially in the setting of aphakia, retinal evaluation should be routinely performed.
Prostaglandin analogues generally do not work well in children. Alpha-adrenergic agonists, such as brimonidine, are associated with extreme fatigue and somnolence in children and should be avoided.3 Although acetazolamide can be used in children, it is not an ideal long-term medication due to renal and hepatic side effects and growth suppression.3
For cases presenting with concomitant inflammation, anti-inflammatory agents may be combined with anti-glaucoma treatment.
Surgical Treatment
When medical management fails, surgical intervention is necessary. Surgical options include angle surgery, trabeculectomy with or without antifibrotics, glaucoma drainage devices (valved or nonvalved), and cyclodestructive procedures. Laser trabeculoplasty has not been found to be effective for managing OAG in children.
Angle surgery. These procedures, which include trabeculotomy or goniotomy, have a variable success rate in glaucoma associated with aphakia or pseudophakia when compared with primary congenital glaucoma; success rates have been reported between 16% and 57%.3
Trabeculectomy. Historically, trabeculectomy has been the most commonly performed procedure, with reported success rates ranging from 50% to 85%.9 A thick Tenon’s capsule and a more exuberant healing response limit this surgery’s success in children.
Use of mitomycin C increases the success rates but is associated with a higher risk of bleb-related infection. The infection rate is particularly high in children due to limitations in hygiene control.3 As an alternative, the antifibrotic agent 5-fluorouracil used postoperatively has potentially fewer side effects. However, it often requires frequent subconjunctival injections under general anesthesia and, thus, is not a practical approach in this population.3,9
Drainage devices. Another option, glaucoma drainage devices, can avoid the complications related to the use of antifibrotic agents in filtering surgery. Success rates for valved (e.g., Ahmed) and nonvalved (e.g., Baerveldt) devices have been reported between 44% and 87%. It is difficult to make comparisons among these studies, in part because some were conducted with additional topical medications and some without, and different definitions of success were used.3
Complications associated with drainage devices include hypotony, choroidal effusion, tube exposure, motility disturbances, and endophthalmitis.3 Prevention of vitreous incarceration in the tube is important.
Although higher success rates and better side effect profiles have been reported for glaucoma drainage devices than for trabeculectomy, a head-to-head analysis of the long-term outcomes of these surgical interventions will be necessary for definitive comparison. In aphakic patients who use contact lenses for correction, glaucoma drainage devices may be preferable to filtering surgery to reduce the risk of infection.
Other surgical therapies. Cycloablative procedures, such as cyclocryotherapy and cyclophotocoagulation, are useful in refractory cases, but success rates are low over the long term. Often, these procedures require multiple treatments and carry the risk of serious vision-threatening complications. Endocyclophotocoagulation, a more targeted treatment, may reduce the risk of hypotony.3
In cases of pseudophakic pupillary block glaucoma, Nd:YAG iridotomy has been reported to be successful.7
Key Points
Glaucoma associated with aphakia or pseudophakia is a common complication following lensectomy for congenital cataract. Diagnosis relies on clinical examination and ongoing follow-up, as the majority of cases are diagnosed many years after surgery. Although open-angle glaucoma accounts for the large majority of cases, angle-closure glaucoma may occur infrequently.
Medical management is the first-line treatment and has a high success rate. If glaucoma drugs fail, filtering surgery with antifibrotic agents and drainage implants have both proved effective in lowering IOP; however, each has specific complications that require close follow-up. Amblyopia is the major cause of vision loss in children with unilateral cataract or glaucoma. With early recognition and successful management of glaucoma, children can achieve good visual potential.4
___________________________
1 Asrani S et al. J AAPOS. 2000;4(1):33-39.
2 Chen TC et al. Arch Ophthalmol. 2004;122(12):1819-1825.
3 Baily C et al. J Clin Exper Ophthalmol. 2012;3(1):203. doi:10.4172/2155-9570.1000203.
4 Whitman MC et al. Semin Ophthalmol. 2014;29(5-6):414-420.
5 Freedman SF et al. JAMA Ophthalmol. 2015;133(8):907-914.
6 Chen TC et al. Trans Am Ophthalmol Soc. 2006;104:241-251.
7 Vajpayee RB et al. Am J Ophthalmol. 1991;111(6):715-718.
8 Asrani S et al. Ophthalmology. 1995;102(6):863-867.
9 Glaucomas in aphakia and pseudophakic after congenital cataract surgery. In: Mandal AK, Netland PA. The Pediatric Glaucomas. Oxford, U.K.: Butterworth-Heinemann; 2006.
___________________________
Dr. Turlapati is a fellow in pediatric ophthalmology at Children’s National Medical Center in Washington, DC. Dr. Salim is professor of ophthalmology and chief of the glaucoma service at Medical College of Wisconsin in Milwaukee. Dr. Freedman is professor of ophthalmology and chief of the pediatric ophthalmology and strabismus division at Duke Eye Center in Durham, N.C. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.