By Mike Mott, Contributing Writer, interviewing Lisa S. Schocket, MD, Sharon D. Solomon, MD, and Kenneth Taubenslag, MD
Download PDF
Anti-VEGF therapy has become the gold standard for managing sight-threatening retinal diseases such as age-related macular degeneration, foveal edema secondary to retinal vein occlusion, and center-involved diabetic macular edema. As a result, intravitreal injection (IVI) is now the most common ophthalmic procedure in the United States—and among the most common in all of medicine. These trends seem likely to continue, given the aging of the population.
The procedure is seemingly simple but requires many small maneuvers that can introduce inefficiency and compromise sterility along the way. Although the safety profile of IVI is well established, complications can lead to devastating visual outcomes, said Kenneth Taubenslag, MD, at the Vanderbilt University Medical Center in Nashville, Tennessee. “You really need to strive for perfect technique and stay up to date on best practice guidelines because, in the end, IVIs are not as trivial as meets the eye,” he said.
 |
|
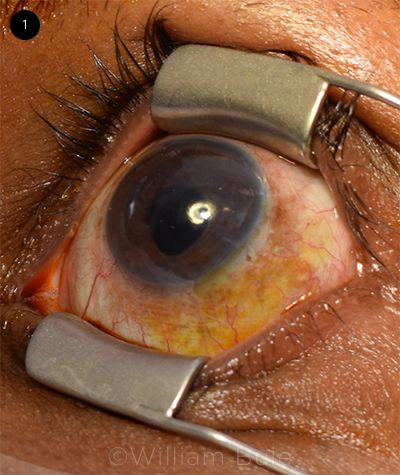
PREP. Dr. Solomon places a lid speculum and uses Betadine on the conjunctiva as well as the eyelids and eyelashes.
|
Taking It Step by Step
In many academic institutions and resident-run clinics, trainees perform a majority of IVIs—learning from faculty everything from proper consent and tray organization to body position and injection location. “For practicing ophthalmologists, reviewing these steps is also important,” said Lisa S. Schocket, MD, at the University of Maryland Medical System in Baltimore. “We perform so many of these injections that we often forget about where the mistakes can be made.”
Knowing the indication. It might seem obvious, but first the physician should determine whether the patient needs to undergo the procedure, said Sharon D. Solomon, MD, at the Wilmer Eye Institute in Baltimore. “Does the patient indeed have active choroidal neovascularization, for example, or center-involved diabetic macular edema that’s causing significant vision loss? Depending on the clinical indication, the patient may do well without IVI treatment,” she said.
Patient consent. Communicating risk is another important step prior to performing the IVI, said Dr. Solomon. The ophthalmologist needs to explain to the patient why therapy is recommended in the first place and to make sure that the patient has a good understanding of the potential complications of the procedure, including the risk of endophthalmitis, retinal tear, vitreous hemorrhage, and puncture of the lens, which can cause premature cataract progression, she said. (For more about informed consent, see omic.com/anti-vegf-drugs-in-adults.)
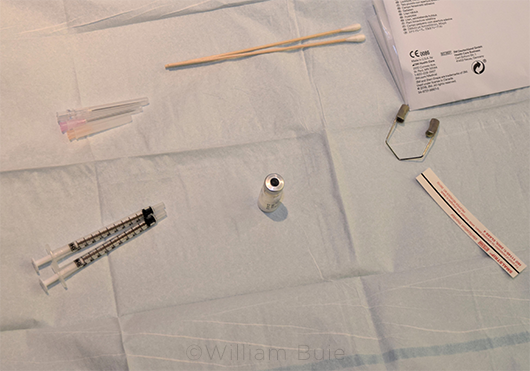
A tidy workspace. Keeping your Mayo stand clean and positioned correctly is also critical, said Dr. Schocket. “I make it very clear to our residents from day one that they must keep an organized workspace for two important reasons. One, you’re more likely to make a mistake if you’re trying to navigate chaos, and two, you want to convey to the patient that they are in a clean and organized environment and that you are serious about preventing infection.” If a patient develops a postop complication, it’s important to know that you did everything possible to prevent infection and that you gave the patient a sense of confidence in your care, she said.
Separating the syringes. If your anesthetic preference is a subconjunctival lidocaine injection rather than topical jelly, be sure that you draw (and apply) the agent before drawing the anti-VEGF medication in a second syringe, said Dr. Schocket. “Don’t leave two drawn syringes on your tray. This is something that seems obvious but, remember, we’re trying to eliminate any variable that can introduce risk.” Drawing up the two syringes at once can lead to accidentally swapping the order of the injections.
Body positioning. Placing yourself in proper relation to the patient is another often overlooked aspect of performing an IVI. “Avoid having the patient sit upright,” said Dr. Solomon. A recumbent position is more comfortable for the patient, and it allows the ophthalmologist to have more control over the patient’s body. “It also leads to better adherence of the povidone-iodine [Betadine] antiseptic to the eye’s surface since nothing will be running down the patient’s face,” she said.
For reasons of body positioning, Dr. Schocket prefers injecting inferotemporally in case the patient lifts an arm or leg toward the eye. Standing next to the patient’s body rather than at the head of the bed allows the physician to prevent the patient from moving or reaching forward.
Precise injection site. To prevent lens touch, the ophthalmologist should aim for injection 4.0 mm behind the limbus in phakic patients and 3.5 mm for pseudophakic and aphakic eyes, said Dr. Schocket. “Injecting the drug too close to the lens will cause an instant cataract, while an injection that is too posterior can cause a retinal tear,” she said. And for maximum safety, she instructs her residents to place the needle fully into the vitreous cavity before injecting—not halfway or a quarter of the way.
 |
|
MAYO STAND. An organized tray may help reduce error and may demonstrate to the patient your seriousness about preventing infection, said Dr. Schocket.
|
Preventing Endophthalmitis
The most feared complication of IVI is infectious endophthalmitis. Incidence rates are low, ranging from 0.019% to 0.09%, but visual prognosis is poor despite current treatments.1 That’s why it’s especially important to be aware of the most up-to-date clinical protocols for IVIs—as they’re in a constant state of refinement, said Dr. Taubenslag.
Lid retraction. Eyelids and eyelashes are significant sources of infection, and the lid speculum is the most common tool for avoiding any contamination of the procedural needle. But is it the only way? Not necessarily, said Dr. Taubenslag. To prevent the eyelids and eyelashes from coming in contact with the injection site, he often performs intravitreal injections with manual eyelid retraction. He noted that many patients prefer this, as clumsy insertion or removal of the speculum can be uncomfortable and even cause corneal abrasions in rare cases. Nevertheless, he said, “I would always encourage use of the lid speculum for procedures requiring anterior chamber paracentesis, for tap and inject, and for those patients who have difficulty keeping their eyes open or following instructions.”
Betadine. Drs. Schocket and Solomon are strong proponents of lid speculums, especially in combination with Betadine. “In my experience, these are the two most important ways to prevent infection,” said Dr. Solomon. For the IVI clinical trials in which she has participated, she noted that patients with Betadine allergies are excluded. “It’s that mandatory,” she said. For injection prep, she places the sterile lid speculum inside the eyelid and applies 5% Betadine to the conjunctiva and 10% to the eyelids and eyelashes (Fig. 1). Because the antiseptic can be caustic, she thoroughly rinses the eye with sterile saline solution following the procedure.
Lidocaine use. To gel or not to gel is a controversial topic when considering the delivery of lidocaine. Recent studies have shown that the use of lidocaine jelly or tetracaine gel may increase the risk of endophthalmitis following IVI.2 The reasoning is that the gel can act as a barrier to antisepsis, preventing the Betadine from coming in contact with the conjunctiva and therefore promoting bacterial survival prior to the injection, said Dr. Schocket.
Other studies have shown that sub-conjunctival 2% lidocaine/0.1% methylparaben may actually reduce the incidence of endophthalmitis after IVI due to the methylparaben’s intrinsic antibacterial properties. It’s also believed that the subconjunctival lidocaine dilutes any pathogens adhering to the injection needle, retarding the entry of bacteria from the ocular surface through the injection track.3
“I’ve certainly witnessed cases of endophthalmitis following the use of lidocaine gel,” said Dr. Taubenslag. However, he pointed out that it has advantages. For example, it can be applied by staff and therefore may help with patient flow in a busy clinical environment. “What is critical is to ensure that the Betadine is applied prior to the application of the gel anesthetic and once again prior to injection,” he said.
Masking and draping. The COVID-19 pandemic has created a need for personal protective equipment for both physicians and patients. Early advocates assumed that universal mask protocols would also be beneficial for reducing risk of infection during IVIs. However, the latest research has turned many of these assumptions upside down.
For example, one recent study showed no significant difference in a patient’s risk of endophthalmitis when physician face mask use was compared with a strict no-talking policy during the procedure.4 In addition, a number of other studies have concluded that patients wearing certain face masks during IVIs may actually be at a higher risk of endophthalmitis—due to the masks’ redirection of exhaled air (and oral flora) up toward the injection site.1,5
Dr. Schocket experienced this surprise firsthand. “I am very adamant about infection control. Around 10 years ago, I took what I thought to be an extra step in the risk reduction process and required my patients to wear masks during any IVI procedure,” she said. Subsequently, her rates of endophthalmitis increased. “I was making infection risk worse by redirecting the patient’s breath toward the eye,” she said. Now that all patients are masked due to COVID-19, she said that it is crucial to either tape the upper edges of the mask to create a seal or use an adhesive surgical drape around the eye that is being injected.
Cataract Surgeons, Beware
A growing body of evidence suggests that even a single prior IVI can raise the risk of posterior capsular rupture during cataract surgery, and this risk significantly increases in patients who have received more than 10 injections.1
With this in mind, cataract surgeons should ask patients about any history of intravitreal therapy, said Dr. Taubenslag. “Some of these eyes won’t demonstrate any clear signs of preoperative posterior capsular abnormalities on the slit-lamp exam. But the surgeon should still inspect for signs of trauma, looking carefully for linear posterior capsular defects or opacities.” If there is suspicion of capsular trauma, he advised considering hydrodelineation rather than hydrodissection and being prepared to place a sulcus lens if necessary.
__________________________
1 Nagar AM et al. J Cataract Refract Surg. 2020;46(2):204-208.
|
Patient Takeaways
Because IVIs are used to treat so many retinal diseases, there’s a tremendous burden on ophthalmologists to perform the safest in-office procedure possible. And the risk of complications doesn’t end when the patient walks out the door. That’s why Dr. Solomon recommends handing out documentation of warning signs and symptoms. “Especially if this is their first injection, it’s good practice to provide your patient with a checklist of troublesome symptoms—decreased vision, flashes of lights, floaters, shadows, infection, anything new that seems unusual from baseline. This will ensure that in the rare event a complication does arise, you and your patient can quickly address the situation.”
__________________________
1 Hadayer A et al. Retina. 2020;40(9):1651-1656.
2 Stem MS et al. Ophthalmol Retina. 2019;3(1):3-7.
3 Tustin A et al. Retina. 2014;34(5):935-942.
4 Patel SN et al. Am J Ophthalmol. 2020;222:194-201.
5 Patel SN et al. Am J Ophthalmol. 2021;223:178-183.
__________________________
Dr. Schocket is an associate professor and chief of the retina division at the University of Maryland Medical System in Baltimore. Relevant financial disclosures: None.
Dr. Solomon is a professor of ophthalmology at the Wilmer Eye Institute in Baltimore. Relevant financial disclosures: None.
Dr. Taubenslag is a second-year fellow and instructor in ophthalmology and visual sciences at the Vanderbilt University Medical Center in Nashville, Tenn. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Schocket Spouse works for AstraZeneca: E.
Dr. Solomon None.
Dr. Taubenslag None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|