By Sarita Dave, Paula Wynn, MD, and Randy H. Kardon, MD, PHD
Edited by Thomas A. Oetting, MD
This article is from March 2008 and may contain outdated material.
When we first saw Jack Duckworth,* he was 76 years old. He told us that he had noticed a filmy discharge in his eyes when reading or watching TV. His past ocular history was notable for cataract surgery in both eyes eight years previously. We treated him for blepharitis and meibomian gland dysfunction and instructed him to perform warm compresses and lid scrubs. He didn’t try the lid hygiene methods but seemed to tolerate the blurriness by simply wiping his eyes with a tissue when he turned on the TV.
Blurry Vision and Seeing Double
Over the next five years, we saw him at regular intervals, and he always had the same complaint of intermittent blurry vision in the evenings.
On one visit, he reported episodes of double vision. This occurred in the evening, while he was watching TV, and he described it as intermittent and horizontal.
His symptoms seemed consistent with monocular diplopia, and artificial tears were recommended.
The complaint of diplopia did not resurface until almost two years later. This time, he initiated the clinic visit because his double vision had become more frequent and more constant. He told us that he had been suffering binocular, horizontal and vertical diplopia for two months, and that it was more common later in the day. He felt that his visual acuity was otherwise stable, and he denied any orbital pain with eye movement or headache.
On exam, his visual acuity was indeed stable and there was no relative afferent pupillary defect. The ocular motility exam revealed mild limitation of abduction and depression of the right eye and mild limitation of adduction and abduction of the left eye. Orthoptic measurements revealed a small, comitant right hypertropia and a V-pattern exotropia. No lid ptosis was appreciated at the beginning of the clinic visit, but after an exhaustive exam with remeasurement of the hypertropia/exotropia, the right upper lid became increasingly ptotic.
Differential Diagnosis
Mr. Duckworth’s intermittent episodic diplopia with vertical and horizontal image separation suggested a differential diagnosis of thyroid eye disease; myasthenia gravis; mild skew deviation with accompanying internuclear ophthalmoplegia; myositis; or superior oblique palsy.
However, if he had a superior oblique palsy or Graves’ orbitopathy due to restriction, we would expect that to be accompanied by an esotropia, not an exotropia. Lack of pain made a diagnosis of myositis or orbital inflammation unlikely.
Blood samples were drawn to evaluate levels of thyroid-stimulating hormone and anti-acetylcholine receptor (anti-AChR) antibodies. We scheduled Mr. Duckworth to come in the next week for orbital ultrasound to evaluate him for possible thyroid-related orbitopathy and for a follow-up evaluation of his measurements.
One Week Later
When we next saw Mr. Duckworth he did not complain of any change in his diplopic symptoms, but the changes in his ocular motility exam were profound.
The right upper eyelid was very ptotic and, although he continued to have a mild right hypertropia, his horizontal deficits had progressed into a significant adduction deficit resembling an internuclear ophthalmoplegia.
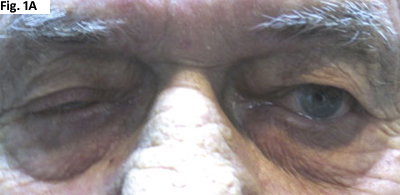
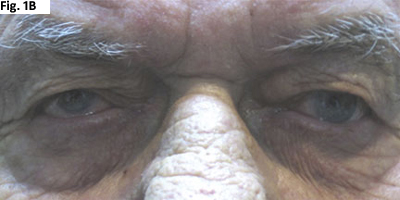
Throughout his examination, the misalignment and ptosis seemed to worsen with repeated testing, but resolved with minutes of rest (see Figs. 1A and B).
In addition, a Cogan’s lid twitch was elicited: When returning to primary position from downward gaze, the eyelid briefly retracted and “twitched” before settling into its ptotic position.
The orbital ultrasound, obtained before his examination that day, was normal with symmetric extraocular muscles of normal thickness. The level of thyroid-stimulating hormone also was normal.
In summary, Mr. Duckworth had a right hypertropia, a left adduction deficit with exotropia and right upper lid ptosis, which demonstrated fatigability and variability, and Cogan’s lid twitch. Our level of suspicion for myasthenia gravis grew substantially.
|
|
|
REST TEST. The patient’s misalignment and ptosis worsened with repeated testing (1A), but resolved with minutes of rest (1B).
|
|
|
|
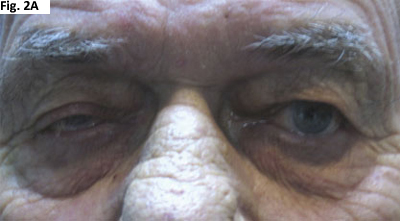
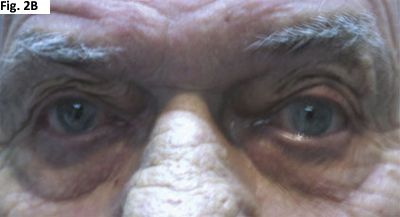
EDROPHONIUM TEST. His right upper eyelid ptosis (2A) improved after edrophonium had been administered (2B).
|
Discussion
Myasthenia gravis (MG) is an autoimmune disorder that affects acetylcholine receptors on postsynaptic neurons at the neuromuscular junction. In people aged 20 to 40, women are three times more commonly affected; in 50- to 60-year-olds, men are more commonly affected.
Onset is typically insidious as the weakness may initially be vague and episodic. The ocular muscles are often the first affected, causing intermittent ptosis and diplopia that remits with rest. Generalized disease develops in 75 percent of patients, involving facial and nuchal muscles, erector spinae and limb musculature. In severe cases, weakness of the diaphragm and chest may produce dyspnea, requiring mechanical ventilation and aggressive treatment.1 The disease can present with other autoimmune disorders, such as thyroid disease, but does not have precipitating factors and is only rarely familial.
The use of particular medications can exacerbate the condition and, in some situations, has caused subclinical cases to become manifest. Penicillamine has been shown to be associated with autoantibody production. Many antibiotics, including aminoglycosides, bacitracin, polymyxins and monobasic amino acid antibiotics (clindamycin and lincomycin), may decrease the production or release of acetylcholine. Worsening of MG has been seen with erythromycin and iodinated contrast dye. Chloroquine, lithium and magnesium affect pre- and postsynaptic transmission. Antiarrhythmic agents (procainamide and quinidine), phenytoin, beta-blockers, calcium channel blockers, cisplatin, phenothiazines and tetracyclines also can worsen the condition.
On physical exam, MG may mimic internuclear ophthalmoplegia and oculomotor nerve palsies. Cogan’s lid twitch may be present, as well as contralateral eyelid droop with elevation of ptotic lid, though the latter is not specific for MG. Cogan’s lid twitch is not diagnostic, but its presence does suggest MG by demonstrating the rapid recovery and easy fatigability of ocular musculature.
There is no single diagnostic criterion, but clinical presentation, immu-nologic studies and simple office tests can provide a basis for diagnosis.
Diagnostic office tests include the ice test (resolution of ptosis after an ice pack has been applied to the eyelid for two minutes; the positive and negative likelihood ratios are 24 and 0.16, respectively), rest test (resolution of ptosis after two minutes of rest; 16 and 0.52), sleep test (resolution of ptosis after eyes have been closed for 30 minutes; 53 and 0.01) and anticholinester-ase test (see “Mr. Duckworth’s Case,” below; 15 and 0.11).2, 3, 4, 5
Anti-acetylcholine receptor (anti-AChR) antibodies have specificity for both generalized and ocular MG (97 to 99 percent), but have low sensitivity for the latter (44 to 66 percent). Typically, serum is assayed for binding, blocking and modulating anti-AChR antibodies, as the sensitivity and specificity of each is dependent on the extent of disease. The binding antibody is positive in 90 percent of patients with generalized MG and only 70 percent with ocular disease. Blocking antibody is positive only in 60 percent with generalized and 50 percent with ocular MG.1
An antibody against muscle-specific tyrosine kinase (anti-MuSK) has been recently discovered in patients who were otherwise classified as seronegative. Its presence correlates with more severe disease and increased cortico-steroid requirement.6
Repetitive nerve stimulation (RNS) and single fiber electromyography (EMG) are additional methods that can be used to diagnose ocular MG. The sensitivity and specificity of RNS vary widely depending on the disease’s severity and on the muscle group tested. In one study, the specificity (94 percent) was similar to the other tests described while the sensitivity is very low (29 percent). Single-fiber EMG has similar heterogeneity but has improved sensitivity (97 percent sensitivity and 92 percent specificity when testing the orbicularis oculi, and 86 percent sensitivity and 73 percent specificity when testing the frontalis).3
MG has been associated with other autoimmune diseases, including Graves’ ophthalmopathy. In addition, 50 to 80 percent of affected individuals have thymic lymphofollicular hyperplasia, a likely source of causative aberrant T cells.2
For these reasons, individuals diagnosed with MG should be evaluated for thyroid disease and have chest CT or MRI to assess for thymoma.
MG frequently involves ocular muscles at onset and may or may not progress to systemic symptoms. More than 50 percent may develop generalized MG, typically within two years of initial diagnosis.7Progression is more likely in individuals over age 50.1
Ocular MG may or may not respond well to treatment. Some patients do respond to pyridostigmine (Mestinon), an oral anticholinesterase, but many do not. Unresponsive patients with ocular MG may respond well to oral prednisone. Usually, this is given in a gradually increasing dose (to reduce the likelihood of paradoxical worsening) up to 60 to 80 mg per day of oral prednisone for at least a month before slowly tapering to find a maintenance dose.
In cases where steroid sparing is a long-term goal, immunosuppressive agents such as Imuran (azathioprine) can be instituted.
Spontaneous remission is seen in 18 percent of individuals, while 33 percent have symptomatic improvement with medications alone.
Thymectomy results in improvement in 85 percent of individuals and remission in 38 percent, with less overall need for anticholinesterase.2
Treatments
Consider the following treatment options for ocular MG:
- Anticholinesterases (e.g., pyridostigmine, neostigmine)
- Thymectomy when thymoma present
- Immunosuppressants, such as prednisone, Imuran shown to delay progression from ocular MG to generalized MG1
- Plasmapheresis
- High-dose intravenous immunoglobulin
___________________________
1 Lee, A. G. Curr Opin Ophthalmol 1996;7:39–41.
|
Mr. Duckworth’s Case
Mr. Duckworth’s anti-acetylcholine receptor antibody panel had not yet been completed when he started demonstrating improved motility. His symptoms improved with a rest test, but further assurance of his diagnosis was needed.
A Tensilon (edrophonium, an acetylcholinesterase) test was performed. We used an intravenous butterfly needle to administer 0.4 mg of atropine, which blocks the muscarinic side effects of acetylcholinesterase, followed by 1 ml of normal saline.
We then gave Mr. Duckworth 2 mg of edrophonium chloride and monitored him closely for lacrimation, sweating, bradycardia or change in ptosis or motility.
In this test, the patient is given additional edrophonium chloride in 2-mg increments (maximum dose 10-mg total) until an unequivocal response is observed.
In Mr. Duckworth’s case, we noted an improvement in his right upper eyelid ptosis 60 seconds after we had administered 6 mg of edrophonium (Figs. 2A and B).
The adduction deficit (pseudo-INO) in the left eye also improved dramatically.
His significant improvement strongly supports the diagnosis of MG.
Plan for Mr. Duckworth
Days after Mr. Duckworth’s visit, the acetylcholine receptor blocking antibody was reported as positive. A chest CT with contrast was ordered to evaluate for thymoma, and he was transferred to the neurology service to undergo further evaluation for generalized MG and therapy.
___________________________
* Patient name is fictitious.
___________________________
Ms. Dave is a medical student; Dr. Wynn is a resident and Dr. Kardon is professor of ophthalmology and director of neuro-ophthalmology. All three are at the University of Iowa. Dr. Kardon is also at the VAMC in Iowa City.
___________________________
1 Yanoff, M. Ophthalmology, 2nd ed. (St. Louis: Mosby, 2004),1335–1339.
2 Alper, B. DynaMed 2007 Mar 27.
3 Benatar, M. Neuromuscul Disord 2006;16:459–467.
4 Scherer, K. et al. JAMA 2005;293:1906–1915.
5 Van Stavern, G. P. et al. J Neurol Sci 2007;256:84–85.
6 Deymeer, F. et al. Neurology 2007;68:609–611.
7 Kupersmith, M. J. et al. Arch Neurol 2003;60:243–248.