Download PDF
Research teams around the world are developing electronic surrogates for sight. Here are some of the most promising advances.
Bionic eye research has reached a tipping point: After decades of painstaking exploration and development, 2 retinal visual prostheses are on the market in Europe and the United States, and, as of early 2016, more than 20 teams around the world were actively engaged in this field of inquiry.1
Their efforts are robust enough—and the number of people worldwide with vision impairment is, at 285 million,1 large enough—that the global market for retinal implants is expected to rise to more than $1 billion by 2022.2 And even though investigators are careful to note that the vision provided by these devices is—at least at this time—relatively rudimentary, they are encouraged by the pace of development and the potential applications.
Surgical Strategies
Multiple teams at work means that multiple strategies—and multiple surgical locations for implantation and stimulation—are being pursued. Most of the devices target the retina, and they can generally be characterized as epiretinal, subretinal, or suprachoroidal, depending on the location of their internal components. However, another innovative approach bypasses the retina altogether and involves direct stimulation of the visual cortex.
Epiretinal Placement
Epiretinal prostheses are placed on the anterior surface of the retina, where they stimulate ganglion cells.
Research spotlight: Argus II. This is perhaps the best-known retinal prosthesis, as it was the first one to be approved for use in the United States and Europe. The device, developed by Second Sight, consists of external electronic equipment worn by the user and internal elements that are implanted in and on the eye.3 The external elements include a video camera that is mounted onto a pair of eyeglasses; the internal elements are a 6×10-mm array of 60 electrodes that is tacked over the macula and an electronics case and implant coil attached to a scleral band sutured onto the eye wall. Because of its design, the recipient must use head scanning to acquire visual information.
Intended applications. The Argus II is approved only for patients with outer retinal degenerative disease such as retinitis pigmentosa (RP) and choroideremia. Researchers are now investigating its use in dry age-related macular degeneration (AMD).
Surgical considerations. The inclusion criteria are “quite specific,” said K. Thiran Jayasundera, MD, at the University of Michigan. “You must have bare light perception, and you can’t have optic nerve problems.” In addition, a patient’s axial length measurements must fall within certain parameters, and evidence of intact inner layer retinal function must be confirmed before implantation.
While adverse events have included hypotony, conjunctival dehiscence, conjunctival erosion, and presumed endophthalmitis,3 “adverse events are quite low, actually, with good surgical technique,” said Dr. Jayasundera, who has implanted the device. He noted that proper placement of the electronics case and receiver coil is “critical to the placement of the array internally; the array falls on the macula if these elements are on the right position on the sclera.” He added that “sclerotomy closure is very important, as there can be leakage through an open sclerotomy.”
Current status. The Argus II has been implanted in more than 190 patients worldwide. Results to date indicate that the device remains a reasonably reliable and stable option for patients with advanced RP. In a recent 5-year follow-up of 30 patients who received the Argus,3 it remained in place and functional in 24. Two devices failed, and 3 were explanted because of adverse events (1 patient died of unrelated causes). In functional “follow the door” and “follow the line” mobility testing, patients were able to maneuver with more success when the system was turned on than when it was off.
As for expanded use, initial results in a study of 4 patients with late-stage dry AMD found that central vision function was elicited by the device over the area of subfoveal geographic atrophy.4 And improvements in the device continue: Second Sight is working on a new iteration that will include updated algorithms, Dr. Jayasundera said.
Similar devices in development. The Iris-II epiretinal implant (Pixium Vision) is in clinical trials in Europe. The device, which is also designed for patients with RP, has 150 electrodes and a readily explantable design.
Subretinal Placement
Subretinal devices are placed beneath the retinal pigment epithelium in the photoreceptor layer.
Research spotlight: Alpha-IMS. This prosthesis, developed by Retina Implant in Germany, has a 3×3-mm microchip that is implanted in a subfoveal position. The chip contains 1,500 light-sensitive photodiodes that are joined to microelectrodes.
The Alpha-IMS prosthesis does not use an external camera; instead, the photodiodes are coupled to an external power module that is implanted under the skin behind the ear and amplifies the signals generated by the photodiode array.
One benefit of the Alpha-IMS is that the patient does not need to use head scanning to locate objects; instead, normal eye movements can be used. “The image moves with the eye because the chip is embedded exactly where the photoreceptors ordinarily would lie,” said Robert E. MacLaren, MD, of Oxford University in England. “By scanning an image with saccades, patients can get more information out of it than would be the case with a static image.”
With regard to the implantation procedure, “The surgery is complex, but any high-volume vitreoretinal surgeon should be able to learn it. Help is also needed from a cochlear implant surgeon to place the power supply under the skin behind the ear,” said Dr. MacLaren, who is involved in clinical trials of a newer iteration of the device. For the retinal surgeon, he said, “The most difficult part is positioning the chip underneath the retina, but this is important because in this position the electronic signals imparted by the chip are directed toward the bipolar cells, which takes advantage of the natural retinal processing.”
Intended applications. The Alpha-IMS prosthesis is approved in Europe for patients with RP. A newer version, the Alpha-AMS, is in clinical trials. Dr. MacLaren reported that he is currently implanting the AMS chip—which is larger in size and contains 1,600 pixels—as part of a study funded by the United Kingdom’s National Health Service.
Current status. In 2015, Alpha-IMS researchers reported 12-month results with 29 patients.5 Thirteen of the recipients were able to recognize object shapes and details in daily life, and 8 could localize high-contrast objects but could not recognize shapes or details. “I have seen several patients now who had no light perception suddenly see things again,” Dr. MacLaren said. “Shapes and outlines of objects may seem rudimentary vision for you and me, but for someone who is completely blind, this is a life-changing moment.” He added, “To date, all patients have been able to see things with the chip. Furthermore, there have been no problems at all with the anesthesia, despite it being a long operation.”
So far, 29 patients have received the Alpha-IMS and 15 have received the Alpha-AMS.
Similar devices in development. Stanford researchers have developed a wireless photovoltaic subretinal prosthesis. Recipients will wear goggles that capture images and project them into the eye and onto an implanted photodiode array; the light is then converted into pulsed current, which stimulates inner retinal neurons.6 The prosthesis, known as Prima, is now being prepared for clinical trials in conjunction with Pixium Vision.
Managing Patient Expectations Is Key
Success with a visual prosthesis depends not only on cutting-edge technology and deft surgical skills but also on the human element. As Kari Branham, MS, CGC, put it, patient counseling is “one of the most critical components of the entire process.”
Counseling “begins with an initial telephone screening and continues throughout the postoperative period,” said Ms. Branham, at the University of Michigan, adding that managing expectations is key. “We spend a lot of time on gauging their expectations. If someone says, ‘I want to see my wife’s face again,’ that’s not realistic. We tell that patient, ‘You might be able to tell that your wife is standing in front of you, but you won’t be able to see her face as you once could.’”
Preoperative evaluation includes at least 2 screening visits. In addition to medical evaluation, Ms. Branham said, “We spend a lot of time on education. You’re not going to have the prosthesis put in and then go on your merry way; you have to be committed to making it work and prepared to go through extensive low-vision rehabilitation afterward.”
After this, potential patients are given time to reconsider before agreeing to surgery. “We really want them to go home and think about it and talk to their family first,” Ms. Branham said. The University of Michigan team also recommends that potential patients consult with several people who have had the Argus II implanted and have offered to serve as peer counselors.
After all of this, some potential patients have decided to decline, Ms. Branham said. “Once we described what they could expect, several people weren’t really interested in having it done. But some have told us that they’re willing to wait for the ‘next generation’ to come out.”
To date, the Michigan team has implanted 10 of the Argus II devices, more than any other single site in the United States. And the recipients continue to be happy with their outcomes. “If you’re a fully sighted person, it can be hard to understand this, but people are pretty happy with the amount of vision that they get back with visual prostheses,” Ms. Branham said. “They’re going from nothing to something, and that means a lot to them.”
|
Suprachoroidal Placement
Suprachoroidal prostheses are designed to be placed between the choroid and the sclera. In a variation of this approach, a prosthesis known as the suprachoroidal-transretinal device is implanted between the layers of the sclera rather than in the suprachoroidal space.
Research spotlight: Bionic Vision Australia. Researchers with the BVA Research Consortium have developed a 24-channel prototype suprachoroidal prosthesis that comprises an intraocular electrode array in a 19×8-mm silicone base. “The array has 33 platinum stimulating electrodes and 2 return electrodes, and the outer ring of electrodes are ganged together to enable hexagonal stimulation, meaning that 20 electrodes can be stimulated individually,” said Carla J. Abbott, PhD, with BVA.
In addition, to allow for maximum flexibility in device stimulation, “the array is connected with a helical platinum/iridium lead wire to a percutaneous connector implanted behind the ear,” Dr. Abbott said. “This allows direct stimulation of the intraocular array with external electronics and has been used previously in cochlear implant studies.”
Intended applications. As with other retinal prostheses, the BVA device is intended for patients with outer retinal degenerative disease such as RP or choroideremia. Candidates for current trials must have remaining visual acuity of light perception or less in both eyes.7
Surgical considerations. The suprachoroidal location of this device confers 2 main advantages, Dr. Abbott said. “Firstly, the suprachoroidal surgery is far less challenging than that required for epiretinal or subretinal implants and does not breach the retinal tissue, thus negating the need for a vitrectomy or incisions into the retina. Secondly, due to its position, the device has an excellent safety profile.”
As for drawbacks, she said, “The potential disadvantage to the suprachoroidal location is that the electrodes are 250 to 400 μm further away from the target retinal ganglion cells than in an epiretinal implant,” and more electrical current needs to be delivered to stimulate phosphenes. However, studies to date with the BVA device have shown that the retina can be safely and effectively stimulated over a relatively good dynamic range.1
Current status. In 2012, BVA researchers implanted the device in 3 patients with end-stage RP.7 This first-in-human trial “showed that the suprachoroidal anatomical position for the array is a viable, minimally invasive, and relatively straightforward location for an electrode array,” Dr. Abbott said. After 12 months of monitoring, the BVA team reported that the surgery was safe, with no intraoperative adverse effects. Moreover, the position of the device was stable, with no lateral movement or signs of extrusion, the device was able to provide visual percepts in all 3 patients, and the electrode array continued to function, resulting in an extension of the trial to 2 years.7
BVA researchers are now working on the next version of their device, said Anthony N. Burkitt, PhD, director of BVA. “An implant with 44 channels in a staggered arrangement has been developed and has been undergoing preclinical safety and efficacy testing. Although the electrode array has substantially more platinum disk electrodes than the prototype device, it nevertheless has the same dimensions and will be implanted in the suprachoroidal position using the same surgical procedure,” he said, adding that the 44-channel system is “fully implantable and suitable for long-term use.”
The BVA team says that the preclinical testing has given them confidence that the system is safe for patients and will generate useful visual percepts; a forthcoming clinical trial is planned.
Similar devices in development. Research on similar devices is being conducted at Osaka University in Japan and at Seoul National University in South Korea. The Osaka researchers recently reported that their wide-field dual-array suprachoroidal-transretinal device could theoretically activate a larger visual field.8
The BVA Team
Prof. Robyn Guymer, MBBS, PhD, FRANZCO, as clinical program leader, Dr. Penelope Allen MBBS, FRANZCO, as chief surgeon, and Dr. Lauren Ayton, PhD, as clinical research team leader from the Centre for Eye Research Australia, also contributed to this article regarding the Bionic Vision Australia Consortium prosthesis. The Bionic Vision Australia Consortium would like to acknowledge the contribution of its 5 member organizations (the Centre for Eye Research Australia, the Bionics Institute, NICTA, the University of Melbourne, and the University of New South Wales), along with the 3 partner organizations (The Royal Victorian Eye and Ear hospital, the National Vision Research Institute of Australia, and the University of Western Sydney).
|
Bypass the Retina?
Recent advances in such areas as wireless technology and silicon chip design have renewed interest in—and research on—cortical visual prostheses, which bypass the retina altogether and directly stimulate the brain.
Research spotlight: Gennaris. The Monash Vision Group (MVG) in Australia, led by electrical engineer Arthur J. Lowery, PhD, has developed a cortical visual prosthesis known as the Gennaris bionic vision system. The system consists of a camera on a glasses frame that transmits digital photographic images to a computer that sits on a belt at the patient’s waist, said neurosurgeon Jeffrey V. Rosenfeld, MD, FRACS, at Monash University. The computer converts those images into electrical waveform patterns that are sent to a receiving/transmitting antenna that is situated at the back of the patient’s head. This antenna then transmits the signals and power to wireless ceramic tiles that are implanted into the visual cortex.9
“The tiles are small—9×9 mm—and each houses about 43 microelectrodes that penetrate the surface of the brain,” Dr. Rosenfeld said. Each electrode is capable of generating phosphenes, thus creating patterns of light in the patient’s visual field. Moreover, the MVG researchers will be able to control the electrodes for different patterns of electrical stimulation, Dr. Rosenfeld said. “This variable stimulation pattern may sharpen the image quality,” he said, adding that the first recipients “will probably have 4 tiles implanted, but more tiles could be implanted in the future.”
Intended applications. Dr. Rosenfeld envisions the Gennaris system as a complement to, not a competitor with, the retinal prostheses. “There are quite a few patients with acquired blindness who aren’t candidates for the retinal implants” due to loss of healthy ganglion cells, he said, including those with severe glaucoma and those whose blindness is due to trauma, tumors, or optic nerve atrophy.
Surgical considerations. Although some researchers have tried to stimulate various sites in the brain, notably the lateral geniculate nucleus, the “technical challenges are very high in that scenario,” Dr. Rosenfeld said. In contrast, the Gennaris system can be implanted via what he described as straightforward neurosurgery. “Provided you can identify the primary visual cortex, you can put the device in without too much of a surgical challenge.” Of course, no neurosurgery is truly routine, and concerns have been raised with regard to a number of potential adverse events, including postoperative bleeding, swelling, neurological deficits, and seizures.9
Current status. The Gennaris system is still in preclinical studies, and the MVG group is planning to go to human trials in 2017, Dr. Rosenfeld said. “We’re hoping that once patients get accustomed to the patterns, they will be able to navigate their environment, identify large objects, see people—although we’re not promising facial recognition—and maybe, just maybe, read large print.”
Similar devices in development. Second Sight, the maker of the Argus II, has also developed a cortical visual prosthesis named Orion I. Preclinical testing is ongoing, and the company has reported that it plans to go to human trials in 2017.
Further Reading
Ayton LN et al. Choroidal thickness profiles in retinitis pigmentosa. Clin Experiment Ophthalmol. 2013;41(4):396-403.
Chuang AT et al. Retinal implants: a systematic review. Br J Ophthalmol. 2014;98(7):852-856.
Ghodasra DH et al. Worldwide Argus II implantation: Recommendations to optimize patient outcomes. BMC Ophthalmol. 2016;16(1):52.
Lewis PM et al. Advances in implantable bionic devices for blindness: A review. ANZ J Surg. Published online June 14, 2016. doi:10.111/ans.13616.
Lowery AJ et al. Restoration of vision using wireless cortical implants: The Monash Vision Group Project. Conf Proc IEEE Eng Med Biol Soc. 2015:1041-1044.
Rizzo JF, Ayton LN. Psychophysical testing of visual prosthetic devices: A call to establish a multinational joint task force. J Neural Eng. 2014:11(2):020301.
Shepherd RK et al. Visual prostheses for the blind. Trends Biotechnol. 2013;31(10):562-571.
Stingl K, Zrenner E. Electronic approaches to restitute vision in patients with neurodegenerative diseases of the retina. Ophthalmic Res. 2013;50(4):215-220.
Weiland JD, Humayun MS. Retinal prosthesis. IEEE Trans Biomed Eng. 2014;61(5):1412-1424.
|
Next Steps
Investigators readily acknowledge that bionic vision research still has a long way to go. Even so, they are encouraged by where things stand at this point. Next-generation prostheses are expected to offer improvements in quality of image resolution and an expanded field of view, for instance. And in a best-case scenario, inclusion criteria might eventually cover not only patients with AMD but also adults with congenital blindness.
“In their current iterations, visual prostheses are spotting tools that may allow patients to see contrast or outlines,” said Dr. Jayasundera. “The devices certainly won’t provide the vision that will allow patients to read or drive; still, a lot of the happiness patients experience [after receiving a prosthesis] comes from shared visual experiences. What a patient might say is, ‘There’s a birthday cake for my grandchild, and I can see the flames of the candles,’ and they know that the grandchild is, at that same moment, seeing those candles.”
___________________________
1 Brandli A et al. Eye Brain. 2016;8:15-25.
2 www.grandviewresearch.com/industry-analysis/retinal-implant-market. Note: This estimate includes the implantable miniature telescope, which is not discussed in this article. Accessed June 30, 2016.
3 da Cruz L et al. Ophthalmology. Published online July 21, 2016.
4 Stanga PE et al. “Argus II electronic epiretinal prosthesis in advanced dry AMD: Safety and feasibility study and preliminary functional results.” Poster D0194, presented at: ARVO; Tuesday, May 3, 2016; Seattle.
5 Stingl K et al. Vision Res. 2015;111(Pt B):149-160.
6 Lorach H et al. Nat Med. 2015;21(5):476-482.
7 Ayton LN et al. PLoS One. 2014;9(12):e115239.
8 Morimoto T et al. “Surgical feasibility of wide-field dual-array suprachoroidal-transretinal stimulation (STS) prosthesis in middle-sized animals.” Poster D0180, presented at: ARVO; Tuesday, May 3, 2016; Seattle.
9 Lewis PM et al. Brain Res. 2015;1595:51-73.
Meet the Experts
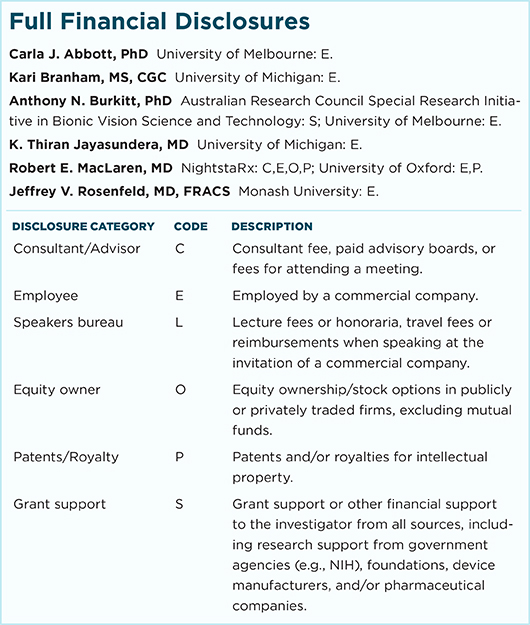
CARLA J. ABBOTT, PHD Research fellow, surgical coordinator, and acting Bionic Eye Clinical Research Team leader at the Centre for Eye Research Australia and Department of Ophthalmology at the University of Melbourne, Australia. Relevant financial disclosures: None.
KARI BRANHAM, MS, CGC Research investigator and genetic counselor at the University of Michigan, Ann Arbor. Relevant financial disclosures: None.
ANTHONY N. BURKITT, PHD Director of Bionic Vision Australia and chair of Bio-Signals and Bio-Systems in the Department of Electrical and Electronic Engineering at the University of Melbourne, Australia. Relevant financial disclosures: Australian Research Council Special Initiative in Bionic Vision Science and Technology: S.
K. THIRAN JAYASUNDERA, MD Assistant professor of ophthalmology and visual sciences at the University of Michigan, Ann Arbor. Relevant financial disclosures: None.
ROBERT E. MACLAREN, MD Professor of ophthalmology at the University of Oxford and consultant ophthalmologist at the Oxford Eye Hospital in Oxford, England, and honorary professor of ophthalmology at the UCL Institute of Ophthalmology and honorary consultant vitreoretinal surgeon at Moorfields Eye Hospital in London. Relevant financial disclosures: None.
JEFFREY V. ROSENFELD, MD, FRACS Director of the Monash Institute of Medical Engineering and senior neurosurgeon in the Department of Neurosurgery at Alfred Hospital in Melbourne, Australia. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.
|