NOTE: This article has been updated since print publication. In the original article, EyeNet incorrectly stated that the biosimilar Razumab had been approved by the FDA and the European Medicines Agency. At time of press, it had not been approved by either agency. The article below removes the mention of these approvals.
Download PDF
Developers of biosimilar drugs have been running a fast-moving global race since the first biologic therapies lost their patents in 2015. And the stakes are only increasing, because drug makers now face a second “patent cliff” as the next round of biologics is slated to come off patent in the next few years.1
In ophthalmology, the push to develop biosimilars is taking place amid a rapidly changing landscape. For instance, a 2020 study found 25 ophthalmic biosimilars in development: four for aflibercept (Eylea), eight for bevacizumab (Avastin), six for ranibizumab (Lucentis), and seven for adalimumab (Humira).1 But just a few months after the study was published, multiple mergers and collaborations—and even outright abandonment of several products—had occurred.
Despite this uncertainty, it’s just a matter of time before ophthalmologists will have the option of using one or more of these novel drugs. And as with any pharmaceutical product, biosimilars will have to clear a series of hurdles, from study design to assessments of safety and efficacy, cost issues, and off-label use, before they achieve broad-based acceptance by clinicians.
Biosimilar Basics
What they are. Technically, biosimilars are molecules with similarity to existing biologic medications, which are known as their innovator biologics or reference medicines. And as with their related biologic drugs, the development of biosimilars is continuing to evolve along with cell line science, protein expression science, and bioengineering.2
But biosimilars offer a compelling alternative to their preexisting counterparts: With biosimilar product development, pharmaceutical companies are able to create drugs similar enough to proven biotherapeutics in safety and efficacy—and they can do so more quickly and at a lower cost.1
For instance, an average innovator biologic costs $1.2 billion to $2.5 billion (in U.S. dollars) and takes roughly 10 to 15 years to develop. In contrast, research and development (R&D) for a biosimilar takes eight to 12 years—and costs $100 million to $200 million.1 Theoretically, those cost savings are then passed on to patients and insurance companies.
What they aren’t. Biosimilars are not generics. Generic drugs are small molecules, relatively simple to duplicate and manufacture. Innovator biologic drugs are 100 to 1,000 times larger than generics and are made up of hundreds of amino acids biochemically married in a particular sequence within a living cellular system.2 Biosimilar versions of biologics are just as complex as their reference medicines.
“A biosimilar is not just a copy of a product like a generic, since much more R&D and scientific study goes into biosimilars than generics,” said Ashish Sharma, MD, at Lotus Eye Hospital and Institute in Coimbatore, India. “They’re highly researched molecules.”
From Bench to Clinic
The road to approval. The FDA’s current standard for approving biosimilars is as follows: “A biosimilar is highly similar to, and has no clinically meaningful differences in, safety, purity, and potency (safety and effectiveness) from an existing FDA-approved reference product. The goal of a biosimilar development program is to demonstrate biosimilarity between the proposed biosimilar product and the reference product, not to independently establish the safety and effectiveness of the proposed product.”3 (See “Safety and Efficacy,” below.)
The road to acceptance. Biosimilars face a unique challenge in that they may be perceived differently than standard medications and thus can trigger a level of skepticism akin to the “nocebo effect,” Dr. Ashish Sharma said. He argues that “Physicians shouldn’t be too skeptical [about] using them, since everything about the active molecule—primary structure, dynamics, pharmacokinetics—has been shown [to be] similar” to the reference medicine.
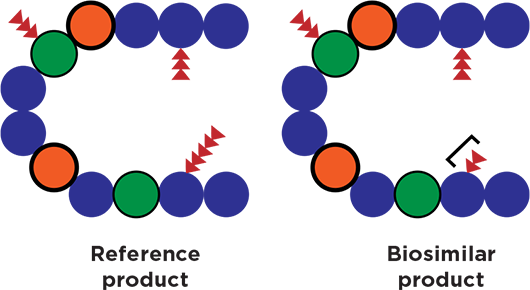 |
|
CLOSE BUT NOT EXACT. Minor variations between reference and biosimilar products may occur in process or structure (bracket). (Adapted from the FDA.)
|
Study Design
How should clinicians assess studies of biosimilars?
Focus on equivalency. Biosimilar drugs aren’t required to be put through phase 1 or 2 clinical trials with one or two years of follow-up data.
“For a biosimilar, you want to show it’s equivalent—it works the same as—the reference product, not worse and not better,” said Neil M. Bressler, MD, at the Wilmer Eye Institute in Baltimore. “You can’t figure out if it’s exactly the same because it’s a biologic agent; that’s why showing that you have an ‘equivalent’ product is the standard.”
An equivalency study sets out to prove that the biosimilar has equivalent biologic activity, Dr. Bressler said. “If you show that the biosimilar acts the same [as the reference medication] out to eight weeks, you don’t need primary outcomes out to one or two years—though during those eight weeks of testing you have to show that there aren’t any safety issues,” Dr. Bressler said.
What does this shorter trial mean for ophthalmologists? “They need to know that the equivalency shown within a [phase 3] randomized clinical trial of the biosimilar and its reference product may be accepted by regulatory agencies as sufficient proof that the biosimilar is the same as the proven product,” said Dr. Bressler.
For instance, this might involve data on anatomic improvement of abnormal retinal thickening or vision outcomes in the short term, he noted. “It may be a new concept to recognize that if you show it’s an equivalent product [for these outcomes] out to four or eight weeks, then you [researchers and clinicians] have confidence that the biosimilar should act as the reference product acted over one or two years.”
Understand nuances. What if a biologic is a monoclonal antibody produced by a cell culture? “The aflibercept that was tested in initial phase 3 clinical trials 10 years ago may not be the identical product in 2020, because the cell cultures that produced the aflibercept in 2010 may be different from the cultures producing aflibercept in 2020, even if the methods for producing those cell cultures are kept constant,” said Dr. Bressler. “With a biosimilar, if it acts the same as the reference product on retinal structure or vision, we’re under the presumption that it will continue to act the same as the reference product after two years.”
In the early days of biosimilars, before the devel-opment of global regulations on these products, several “biomimics”—that is, noncomparable biotherapeutic products—appeared in some countries.2 With guidelines now in place, a biosimilar may even improve on the potency, half-life, or other characteristic of the innovator molecule and become its “biobetter.”2
Safety and Efficacy
Global protocols for biosimilar approval are constantly changing, but in the United States, the FDA generally erects three hurdles: analytical proof of biosimilarity, an animal study on toxicity, and a brief clinical study.4
Burden of proof. “What ophthalmologists need to recognize is that the burden of proof is very different with biosimilars,” said Sumit Sharma, MD, at the Cole Eye Institute in Cleveland, Ohio. “The approval process only needs to show that it’s essentially equivalent in activity and side effect profile, but it doesn’t need to show that it’s exactly the same. Physicians should have a high index of suspicion if they don’t think it will function the same.”
The underlying assumption for each biosimilar is that safety and efficacy were already proven for the reference product. “All the manufacturer has to show is that it’s biosimilar—similar in absorption, in elimination, in levels that it reaches, and in vitro activity,” Dr. Sumit Sharma said. “They don’t actually have to show safety and effectiveness in a biosimilar, so in most cases, it will be safe, but you don’t know for sure.”
Not an identical twin. The FDA looks for data showing similarity with the reference drug in terms of safety and efficacy, but the data will never be exactly the same as that achieved with the original product, he said. “In the antibody production process, a number of phases need critical purification steps to get to a level of purity and avoid toxicity—for example, when you inject in the eye versus subcutaneously—and they’re not required to show the safety side of that,” he explained.
Post-Translational Changes
A variety of post-translational modifications can happen on the way to the clinic. For instance, structural changes can occur between batches of a given biologic, due to oxidation, glycation, and other processes.2 “When a biosimilar antibody is made, the type of bacterial or nonbacterial system it’s made in is proprietary, as are the purification steps taken,” said Dr. Sumit Sharma. Thus, “while the antibody sequence is released to the public, the way it’s made is not.”
Again, not an identical twin. Without a proven blueprint of the entire process, a drug maker is left trying to reverse-engineer a complex, macrocellular product. Almost by definition, a biosimilar may never be exactly the same as its reference product.
Do these minor differences matter? “One of the things we’ve discovered with antibody production is that the process really matters,” Dr. Sumit Sharma said. “If you go back to the beginning of the anti-VEGF era, a number of process improvements were made to reduce inflammation rates. But will all of the biosimilars go through those same process improvements, since they’re not required to do a large study on safety?” After all, as with the initial, detailed manufacturing process for a biologic, all of those early improvements are also proprietary.
In her study, Eva R. Kabir, PhD, put it succinctly: Even small variations in process or structure between a biosimilar and its reference biologic can change the safety and efficacy of a biosimilar.2
True Cost Savings?
As the global race for biosimilars continues, will the promised cost savings materialize? That may depend on where you practice.
Location, location, location. “In India, because we have a big need for cost-effective medications, we’re fine with clinical data from only 120 patients, while Europe would probably need 300 patients,” said Dr. Ashish Sharma. “Biosimilars help solve the problem of lack of insurance in India, Brazil, and other South American countries, where we pay from the pocket.”
Both biologics—and their biosimilar cousins—are made in an expensive, iterative process. While the innovator molecule bears the financial brunt of development, reverse-engineering a biologic to a biosimilar is still costly.
“A company has to spend a lot of money, so it’s not a big price cut, usually about 30% to 40% in India,” said Dr. Ashish Sharma. “It’s also expensive to enter a new country because regulatory requirements are different.”
Moreover, low-resourced countries may already have long-term supplier contracts in place. “A lot of poorer countries already have deals with the big pharmaceutical companies to get access to their medications for much cheaper than what we pay in the United States, sometimes for less than what the biosimilar would cost them,” said Dr. Sumit Sharma. For instance, he said, “When you look at adalimumab [Humira], the average price per dose in the United States is $4,400—but in South Africa, it’s $740.”
Within the United States, some biosimilars may not have dramatic cost savings over their innovator drugs. “With infliximab-dyyb [Inflectra], a biosimilar for infliximab, the eight-week cost is $2,100, whereas it’s $2,600 for the originator infliximab,” said Dr. Sumit Sharma.
“There have been a number of studies looking at the cost savings using biosimilar infliximab in the United States, and while insurance companies may require it, the overall cost saving is not huge,” he said.
Off-Label Use
A specific challenge for ophthalmologists is off-label use of biosimilars from other medical disciplines—for example, infliximab for uveitis.
Ophthalmology is not rheumatology. “The rheumatologic literature finds the biosimilar Renflexis [infliximab-abda] identical to Remicade [infliximab] in terms of activity, but if you look at activity in the eye, we found that it required higher doses to get the same efficacy,” said Dr. Sumit Sharma (see “Research Spotlight”). “Because it’s off label, there were no studies required from the FDA to approve the biosimilar for [ocular] use, so we have no data on its efficacy in the eye.”
All for one—and one for all? A further issue is that “a biosimilar company only has to get approval for one indication, and they’ll get approval for all indications,” said Dr. Sumit Sharma. “You often don’t see the safety signals until you’re looking at hundreds or thousands of patients, so no one has data yet on the safety or efficacy of these medicines. The FDA requires equivalency data in terms of pharmacodynamics and pharmacokinetics. It doesn’t require safety and efficacy data, and that’s the challenge.”
Jennifer K. Sun, MD, MPH, agreed that the core issues are safety and efficacy. “The difficulty with biosimilars is making sure that we have the level of evidence so that we thoroughly understand their efficacy and their safety profile as we start to use them in place of FDA-approved [reference] drugs,” said Dr. Sun, at Harvard. “It may be that while biosimilars are similar to agents accepted for use, there may be small differences in molecular structure or the pathways they influence, so there’s always a possibility of off-target effects that we would want to be aware of.”
Biosimilars at AAO 2020
Alternatives to ranibizumab. Two posters on ranibizumab biosimilars were presented at AAO 2020 Virtual:
- Holz FG et al. COLUMBUS-AMD: Efficacy and safety of FYB201, a proposed biosimilar to ranibizumab, in nAMD. Poster ID# Po387.
- Bressler NM et al. Phase 3 RCT comparing SB11 (ranibizumab biosimilar) with ranibizumab in nAMD: One-year results. Poster ID# Po393.
Both posters are available on demand at aao.org/2020.
|
Looking Ahead
What should you expect in the near future?
Advent of anti-VEGF biosimilars. “The biggest change will happen when the anti-VEGF biosimilars enter the market in the next three to five years,” said Dr. Sumit Sharma.
Dr. Sun agreed. In ophthalmology, “a lot of what drives the biosimilar question has to do with the financial burden of anti-VEGF treatment,” she said. “Anything that changes the ability of patients to pay for these medications, with similar safety and efficacy, will be a key driver of how they get used.”
Need for comparative effectiveness studies. In addition, a different kind of research is needed, said Dr. Sun, who serves as one of the chairs of the DRCR Retina Network, a collaboration of clinical research sites for retinal disease. “It’s going to be critical as these biosimilars come down the pike, both for clinicians and patients, to have good-quality comparative effectiveness studies.”
Dr. Sun suggested several models for high-quality studies: “Comparative effectiveness studies—like the network’s Protocol T (for diabetic macular edema), as well as the CATT study and Ivan study (for neovascular AMD)—have really been essential for us to be able to make individual treatment decisions between medications in these very common retinal diseases with enormous public health impact.”
Need for MD awareness. Given the rapidly expanding pipeline of biosimilars, physicians will be challenged to stay up to date—and to do so, they will need evidence from well-designed studies. “That’s why it’s critical for federal and foundation funding to do these objective comparative studies, which may not be the primary interest of any one specific industry player,” Dr. Sun said.
Of note, information on biosimilars is available on the FDA’s website (www.fda.gov). At time of press, 28 biosimilars had been approved (search for “Biosimilar Product Information”).
 |
|
COMING SOON? Biosimilars for treating wet AMD are garnering considerable research attention. This image was originally published in the ASRS Retina Image Bank. John W. Kitchens, MD. Extensive Submacular Hemorrhage. Retina Image Bank. 2014; Image Number 18055. © The American Society of Retina Specialists.
|
The Bottom Line
Will biosimilars live up to their promise? While the answer is unknown, it’s clear that expert opinions on the pros and cons of biosimilars are as varied as the biotherapeutics themselves—and a number of issues remain to be resolved.
“Having biosimilars is a fantastic idea, but I don’t think [the way that] the approval process, the safety data process, and the pricing have turned out has been enough of a boon in the U.S. market as was hoped for,” Dr. Sumit Sharma concluded.
___________________________
1 Sharma A et al. Br J Ophthalmol. 2020;104(1):2-7.
2 Kabir ER et al. Biomolecules. 2019;9(9):410.
3 www.fda.gov/drugs/biosimilars/biosimilar-development-review-and-approval. Accessed Nov. 10, 2020.
4 Sharma A et al. Clin Ophthalmol. 2018;12:2137-2143.
Research Spotlight
Several biosimilars with ophthalmic potential:
Razumab (Intas) is the first biosimilar of ranibizumab to be available on a global basis. It was approved by the Drug Controller General in India in 2015.
Use for wet AMD. In India, Shashikant Sharma et al. evaluated the long-term use of Razumab injections across 17 sites in the RE-ENACT 2 study.1 The researchers evaluated 103 patients with wet AMD. Improvements were noted in all parameters, including best-corrected visual acuity (BCVA), central subfield thickness, intraretinal fluid, and subretinal fluid. No significant changes in intraocular pressure occurred, and there were no new safety concerns.
Use for other indications. Also in India, Ashish Sharma et al. retrospectively compared outcomes of patients switched from ranibizumab to Razumab.2 This study involved 20 patients with wet AMD, retinal vein occlusion, and diabetic macular edema. No clinical signs of immunogenicity or change in efficacy were noted with the biosimilar.
Renflexis (infliximab-abda; Merck) is one of the biosimilars of infliximab.
Use for uveitis. In the United States, Deaner et al. retrospectively evaluated the frequency of ocular flares in patients with noninfectious uveitis who were switched to Renflexis for nonmedical (i.e., insurance coverage) reasons.3
The researchers assessed 17 patients. The frequency of new or worsening ocular flares increased when patients were switched to the biosimilar, especially within the first 90 days. Most of the ocular flares resolved with increased dosage of Renflexis.
Tumor necrosis factor (TNF)-alpha inhibitors. The biosimilars in this category include Imraldi (adalimumab-xxxx; Biogen), a biosimilar of adalimumab. (Note: The suffixes for Imraldi and Flixabi, below, had not been assigned at time of press.)
Use for uveitis. In Italy, Fabiani et al. compared outcomes of patients switched to biosimilar TNF-alpha inhibitors from their originators.4 Biosimilars evaluated included Imraldi, Flixabi (infliximab-xxxx; Biogen), and Inflectra (infliximab-dyyb; Pfizer). This study involved 37 patients with noninfectious uveitis. No statistically significant differences were noted in frequency of flares, BCVA, frequency of uveitic macular edema, and daily corticosteroid intake.
___________________________
1 Sharma S et al. Ophthalmol Ther. 2020;9:103-114.
2 Sharma A et al. Eye (Lond). 2020;34(6):1008-1009.
3 Deaner JD et al. Am J Ophthalmol. Published online Aug. 11, 2020.
4 Fabiani C et al. Front Pharmacol. 2019;10:1468. doi:10.3389/fphar.2019.01468.
|
Meet the Experts
Neil M. Bressler, MD The James P. Gills Professor of Ophthalmology at the Wilmer Eye Institute, Johns Hopkins University School of Medicine in Baltimore and editor-in-chief of JAMA Ophthalmology. Relevant financial disclosures: Bayer: S; Biogen: S; Genentech/Roche: S; Novartis: S; Regeneron: S; Samsung Bioepis: S. (Note: All research income is paid to the Johns Hopkins University School of Medicine.)
Ashish Sharma, MD A vitreoretinal specialist at Lotus Eye Hospital and Institute in Coimbatore, India. Relevant financial disclosures: Allergan: C,L; Bayer: C,L; Intas: C,L; Novartis: C,L.
Sumit Sharma, MD A retina specialist at the Cole Eye Institute at Cleveland Clinic and assistant professor of ophthalmology at Case Western University’s Lerner College of Medicine in Cleveland, Ohio. Relevant financial disclosures: Alimera: C; Allergan: C,S; Bausch + Lomb: C; Clearside: C; EyePoint: C,S; Genentech/Roche: C,S; Regeneron: C,S.
Jennifer K. Sun, MD, MPH Associate professor of ophthalmology at Harvard Medical School and chief of the Center for Clinical Eye Research and Trials at the Joslin Diabetes Center in Boston. Relevant financial disclosures: Boehringer Ingelheim: S; KalVista: S; Novartis: S; Novo Nordisk: C,S; Roche: C,S.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Bressler Bayer: S; Biogen: S; Genentech/Roche: S; Novartis: S; Regeneron: S; Samsung Bioepis: S. (Note: All research income paid to the Johns Hopkins University School of Medicine.)
Dr. Ashish Sharma Allergan: C,L; Bayer: C,L; Intas: C,L; Novartis: C,L.
Dr. Sumit Sharma Alimera: C; Allergan: C,S; Bausch + Lomb: C; Clearside: C; Eyepoint: C,S; Genentech/Roche: C,S; Gilead Sciences: S; Ionis: S; Regeneron: C,S; Santen: S.
Dr. Sun Adaptive Sensory Technology: S; Boehringer Ingelheim: S; Boston Micromachines: S; Kalvista: S; Novartis: S; Novo Nordisk: C,S; Optovue: S; Roche: C,S.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|