Download PDF
A review of outcomes in more than 100,000 patients suggests that one of the novel oral anticoagulant medications (NOACs) on the market might be less likely than warfarin to cause ocular hemorrhages.
Outcomes better with edoxaban. A network meta-analysis of findings from 12 randomized controlled clinical trials found a statistically significant reduced risk of ocular bleeding in anticoagulated patients who took edoxaban when compared to warfarin (odds ratio: 0.59, confidence interval 0.34-0.98).1 Traditional meta-analyses, using pooled pairwise and subgroup comparisons, also supported this conclusion (both p = 0.02).
The authors hypothesized that the molecular inhibition characteristics of edoxaban and warfarin might explain the difference in bleeding outcomes. “In the case of edoxaban . . . it can be speculated that the direct inhibition of Factor Xa confers enhanced control over the coagulation cascade; in contrast, warfarin targets factors II, VII, IX, X and regulatory factors protein C, S, and Z, offering poorer pharmacodynamic precision,” they wrote.
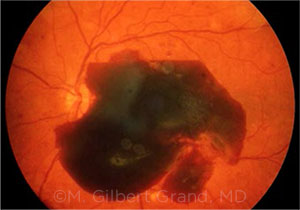 |
BLEEDING. This image shows a preretinal subhyaloid hemorrhage.
|
Equivalent outcomes with other NOACs. No statistically significant reductions in risk were found with 3 other available NOACs (rivaroxaban, dabigatran, and apixaban). “There was a nonsignificant trend toward apixaban having more intraocular bleeding adverse events compared with warfarin, whereas dabigatran and rivaroxaban appeared to have similar intraocular bleeding complications profiles relative to warfarin,” the researchers wrote.
Study limitations. However, the possible ophthalmic lessons from this review study are limited because of deficiencies in the underlying data, commented M. Gilbert Grand, MD, of The Retina Institute in St. Louis.
No sham control. One difficulty is that there is no sham control, Dr. Grand cautioned. The researchers did not “calculate how many people get ocular bleeding because of preexisting ocular disease, without taking warfarin and the NOACs.”
No definition of hemorrhage. In addition, they did not define intraocular hemorrhage, Dr. Grand said. For instance, subconjunctival hemorrhages were included in a list of potential intraocular bleeds. “So we don’t know what type of events they were really analyzing.”
Need for clarification. The study does support the conclusion that spontaneous ocular hemorrhages occur rarely in patients undergoing anticoagulation therapy, Dr. Grand said. However, it “does not add any data describing the risk of hemorrhage in anticoagulated patients who undergo intraocular surgery,” he said. “So this dataset, while valuable in itself, does not provide insights for ophthalmologists who must make decisions as to whether to continue or discontinue anticoagulation at the time of ophthalmic surgery.”
Fortunately, there are published data from earlier research on the safety of vitreoretinal surgery in patients taking warfarin2 or the NOACs,3 Dr. Grand noted.
Need for further research. This study also raises an issue worthy of further examination, Dr. Grand pointed out. “There are certain ophthalmic diseases, such as exudative age-related macular degeneration or proliferative retinopathies, in which patients bleed spontaneously whether or not they’re taking anticoagulants,” he said. “What this study does not determine, and what we need to know, is whether anticoagulation therapy in those patients increases their risk of hemorrhages.”
—Linda Roach
___________________________
1 Phan K et al. Br J Ophthalmol. Published online June 20, 2018.
2 Dayani PN, Grand MG. Arch Ophthalmol. 2006;124(11):1558-1565.
3 Grand MG, Walia HS. Retina. 2016;36(2):299-304.
___________________________
Relevant financial disclosures—Dr. Grand: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Beltran Foundation Fighting Blindness: S; NIH/NEI: S; Research to Prevent Blindness: S; The Shaler Richardson Professorship Endowmment: S; University of Pennsylvania: P. Dr. Lewin: NEI: S; University of Florida: P.
Dr. Grand None.
Dr. Grönlund Agreement Concerning the Research and Education of Doctors (Gothenburg): S; Bayer: L; Cronqvists Foundation: S; De Blindas Vänner: S; Gothenburg Medical Society: S; Swedish Society of Medicine: S.
Dr. Hsu Roche/Genentech: S; Ophthotech: C,S; Santen: S.
Dr. Lewis NEI: S; University of Florida: P.
Dr. Obeid None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review