By Linda Roach, Contributing Writer, interviewing Pravin U. Dugel, MD, K. Bailey Freund, MD, Andreas K. Lauer, MD, and Nicolas A. Yannuzzi, MD
Download PDF
At the beginning of this year, brolucizumab (Beovu) looked like it was set to follow a familiar pattern: A new anti-VEGF drug successfully navigates the long path to FDA approval, and ophthalmologists begin considering whether it can solve lingering clinical issues of treating age-related macular degeneration (AMD).
But on Feb. 23, that scenario changed. In an alert to its members, the American Society of Retina Specialists said that it had received reports of 14 cases of vasculitis following the drug’s approval on Oct. 7, 2019. Of these, 11 were designated by the reporting provider as occlusive retinal vasculitis.1,2
Based on current information, brolucizumab is contraindicated in the presence of active inflammation.2 In addition, if inflammation is noted following injection, close follow-up and imaging are warranted, as some cases of occlusive vasculitis may initially be subtle and others may have a delayed presentation.2
In response to these concerns, Novartis, the drug’s manufacturer, launched an extensive safety review. In early April, the company concluded that “there is a confirmed safety signal of rare adverse events of ‘retinal vasculitis and/or retinal vascular occlusion with or without presence of intraocular inflammation that may result in severe vision loss.’”3 As a result, prescribing information would be updated, the company said.
 |
|
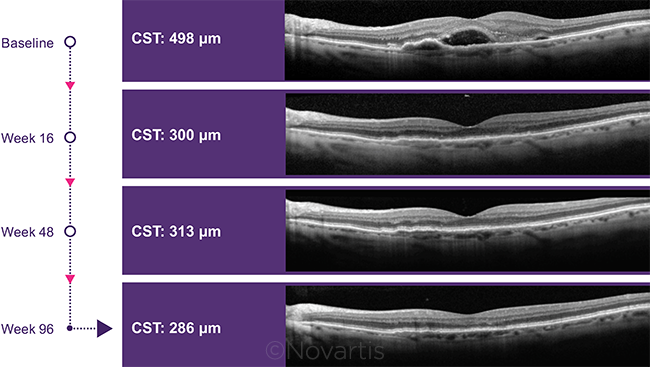
POTENTIAL BENEFIT. Two-year reduction in central subfield thickness in patients who received brolucizumab during the HAWK trial.
|
Why It Was Approved
The drug’s performance in clinical trials suggested that it might help retina specialists address two continuing issues of anti-VEGF therapy—persistent retinal fluid despite treatment and the burden on patients of monthly anti-VEGF injections.
Overview of trials. Two randomized controlled multinational trials of brolucizumab—known as HAWK and HARRIER—were conducted in 1,817 patients with neovascular AMD. In HAWK, patients’ eyes were randomized 1:1:1 to receive brolucizumab 3 mg, brolucizumab 6 mg, or aflibercept 2 mg. In HARRIER, eyes were randomized 1:1 to brolucizumab 6 mg or aflibercept 2 mg.
Visual outcomes. The researchers found that improvements in best-corrected visual acuity (BCVA) obtained with the 6-mg dose of brolucizumab were noninferior to acuity gains with 2 mg of aflibercept, at both 48 and 96 weeks.4 Approximately a third of eyes in both studies gained at least 15 letters of BCVA at 48 weeks. These gains were maintained in the second year, with a mean increase in BCVA of 5.9 letters for brolucizumab 6 mg versus 5.3 letters for aflibercept in HAWK, and 6.1 letters versus 6.6 letters, respectively, in HARRIER.
Sustained drying of fluid. Along with achieving their primary endpoint of noninferiority in BCVA, the trials demonstrated that the 6-mg dose of brolucizumab was better than aflibercept at drying fluid in the retina and at reducing central subfield thickness (CST).
In HARRIER, 24% of the brolucizumab group had intra- and/or subretinal fluid at 96 weeks, compared to 39% of the aflibercept group (p < 0.0001). In HAWK, the comparable figures were 24% and 37%, respectively (p < 0.0001). In addition, fewer eyes receiving brolucizumab 6 mg had fluid beneath the retinal pigment epithelium at 96 weeks: 17% versus 22% for aflibercept in HARRIER, and 11% versus 15%, respectively, in HAWK.
CST reduction. In HARRIER, optical coherence tomography (OCT) scans showed that the mean absolute decrease in CST from baseline at 96 weeks was –198 μm in the brolucizumab subjects and –155 μm in aflibercept eyes (p < 0.0001). In HAWK, the reductions were –175 μm for brolucizumab and –149 μm for aflibercept (p = 0.0057).
Less frequent dosing. Given the treatment burden posed by frequent anti-VEGF injections, many clinicians have hoped that brolucizumab would provide patients with some relief on this front.
Investigators in HAWK and HARRIER tested the efficacy of transitioning patients to a quarterly injection schedule immediately after three initial monthly loading doses, without the gradual treat-and-extend (T&E) process that is being used off-label to lengthen treatment intervals with other anti-VEGF agents.
At one year, half of the brolucizumab patients were successfully on a quarterly injection schedule. Of those patients, 82% in HAWK and 75% in HARRIER were maintained on 12-week intervals for the second year.4 (Brolucizumab recipients who failed the quarterly regimen were treated every eight weeks for the remainder of the trials, without the possibility of extension to every 12 weeks; aflibercept was given at eight-week intervals in both studies.)
Predicting response to therapy. One of the conundrums of anti-VEGF therapy has been in trying to predict which retinas require monthly therapy and which will do well with less frequent injections. The brolucizumab trials showed that, if the drug proved efficacious for a patient early in the trial, their disease likely would remain controlled with 12-week intervals, without a T&E protocol.
Additional thoughts on T&E. As approved, brolucizumab is labeled for three monthly loading doses, followed by an immediate jump to intervals of eight to 12 weeks. This does not allow for the more cautious, gradual T&E protocols that many retina specialists have adopted with other anti-VEGF drugs, said K. Bailey Freund, MD, with Vitreous Retina Macula Consultants of New York in New York City.
“I would be hesitant to use brolucizumab for eyes in which I think it will not be possible to extend to eight-week intervals,” Dr. Freund said. “Also, I prefer to extend gradually; so, after two monthly doses, I might next try a six- or seven-week interval before attempting to extend further.”
Method of Action
Brolucizumab is a recombinant, humanized single-chain antibody fragment—the smallest functional portion of an antibody molecule—that inhibits all isoforms of VEGF-A. It has a molecular weight of 26 kDa, compared to 97-115 kDa for aflibercept (Eylea) and 48 kDa for ranibizumab (Lucentis).1
Because of its small size, the molecule can be delivered into the vitreous at molar doses much higher than previous anti-VEGF drugs, allowing greater penetration into retinal tissue and possibly explaining its extended duration in the eye. For instance, the equivalent molecules per injection is 0.5 for ranibizumab 0.5 mg, 1 for aflibercept 2.0 mg, and 11 for brolucizumab 6.0 mg.1
Evidence from animal studies also suggests that its smaller size may foster quicker systemic clearance.2
___________________________
1 Nguyen QD et al. Ophthalmology. Published online Jan. 17, 2020.
2 Yannuzzi NA, Freund KB. Clin Ophthalmol. 2019;13:1323-1329.
|
Adverse Outcomes
Trial results. Brolucizumab was generally well tolerated in HAWK and HARRIER. However, the results raised some concerns regarding inflammation, as a small number of patients who received brolucizumab experienced uveitis, iritis, and endophthalmitis.2
“In the phase 3 trials, there was a greater proportion of study patients who developed intraocular inflammation—more so than we saw with ranibizumab, aflibercept, or bevacizumab,” said Andreas K. Lauer, MD, at the Casey Eye Institute in Portland, Oregon. Most of the cases of inflammation observed in the trials resolved with no sequelae;1 nonetheless, their occurrence prompted the push to have clinicians track and report their experiences with patient outcomes.2
What’s next? In addition to updating prescribing information, Novartis has informed investigators who are participating in ongoing clinical trials and is amending protocols to include the new safety information.3
Clinicians are encouraged to report any problems to Novartis at www.report.novartis.com or to the FDA at www.accessdata.fda.gov/scripts/medwatch/index.cfm. Retina specialists may also contact www.asrs.org/clinical/adverse-events-reporting/report-an-adverse-event.
What role does COVID-19 play? With the advent of COVID-19, retina specialists are focusing on their most vulnerable patients, including those at greatest risk of vision loss from AMD. As Dr. Freund put it, “Neovascular AMD doesn’t care about the virus, and patients can lose vision if they do not continue treatment.”
Even before COVID-19 became a concern, Dr. Freund said that his large group practice was moving cautiously on adoption of the drug. Initially, he planned to use brolucizumab in two groups of patients: 1) those in whom he was “unable to adequately control exudation with the other anti-VEGFs” and 2) those “currently on a T&E regimen where I would like to extend the injection interval a bit further,” Dr. Freund said. Now, however, he has suspended his use of brolucizumab while he awaits additional findings on the drug’s safety.
Further Clarification Needed
Presuming that brolucizumab regains its footing, a number of issues warrant additional investigation.
Stable disease? Some evidence suggests that brolucizumab might smooth out fluctuations in retinal thickness over time, said Pravin U. Dugel, MD, with Iveric bio, Inc. and based in Phoenix. In post hoc analyses of the brolucizumab data, OCTs taken of brolucizumab eyes did not have the seesaw pattern observed in those taken of aflibercept eyes, he said—and in other trials of anti-VEGF agents, fewer OCT fluctuations correlated with better BCVA, he noted.
If fewer OCT fluctuations are indeed substantiated with brolucizumab, this would be one of the most appealing aspects of the drug for clinicians, Dr. Lauer said. “We know that a consistently sustained level of medication reduces reactivation of the disease, and the clinical picture tends to be more stable,” he said. Support for this comes “from the inflammatory eye disease world, using sustained release corticosteroids, and also from the LADDER study,5 with a port delivery system using ranibizumab,” Dr. Lauer said.
Impact as drying agent. “The data suggest that at the fixed interval dosing regimens evaluated in the clinical trial, brolucizumab appears to be a better drying agent than aflibercept,” said Nicolas A. Yannuzzi, MD, at Bascom Palmer Eye Institute in Miami. “But how much does that matter? Visual outcomes were shown to be noninferior.”
In addition, Dr. Yannuzzi noted, some retina specialists are wary of the idea of completely drying the neovascularization for fear of hastening progression to geographic atrophy.
At any rate, evidence of any benefit of extended dosing and lower treatment burden will have to wait until the drug enters routine clinical practice, said Dr. Yannuzzi.
Good results with PCV. Dr. Freund noted that a potential target population for use of the drug as initial monotherapy would be patients who have polypoidal choroidal vasculopathy (PCV), a subtype of neovascular AMD in which typical soft drusen are often absent but eyes have pachychoroid disease features. This AMD variant is most common in Asian populations. Incidence of this subtype among Asians with neovascular AMD has been estimated to be as high as 50%,6 and there is early evidence that brolucizumab might dry up their lesions quickly, he said.
___________________________
1 aao.org/headline/brolucizumab-s-safety-under-review. Accessed March 12, 2020.
2 Nguyen QD et al. Ophthalmology. 2020. Published online Jan. 17, 2020.
3 www.novartis.com/news/novartis-completes-safety-review-and-initiates-update-beovu-prescribing-information-worldwide. Accessed April 13, 2020.
4 Dugel PU et al. Ophthalmology. 2020;127(1):72-84.
5 Campochiaro PA et al. Ophthalmology. 2019;12 6(8):1141-1159.
6 Takahashi A et al. Ophthalmol Retina. 2018;2(4):295-305.
___________________________
Dr. Dugel served as principal investigator of HAWK and is executive vice president and chief strategy and business officer of Iveric bio. He is based in Phoenix. Relevant financial disclosures: Iveric bio: E; Novartis/Alcon Pharmaceuticals: C; Roche/Genentech: C.
Dr. Freund practices at Vitreous Retina Macular Consultants of New York and is clinical professor of ophthalmology at the New York University School of Medicine, both in New York City. Relevant financial disclosures: Novartis/Alcon Pharmaceuticals: C; Optovue: C; Roche/Genentech: S.
Dr. Lauer is chair of ophthalmology and chief of the Retina-Vitreous Division of the Casey Eye Institute at Oregon Health & Science University in Portland. Relevant financial disclosures: Allergan: S; Biogen: C; Genentech: S; NEI: S; Nightstar/Biogen: C; Oxford BioMedica: S; Regenxbio: C; Sanofi: C.
Dr. Yannuzzi is a fellow in retina and vitreous diseases, an instructor, and chief ophthalmology resident at Bascom Palmer Eye Institute in Miami. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Dugel AbFero Pharmaceuticals: C; Acucela: C; Adverum Biotechnologies: C; Aerie Pharmaceuticals: C; Aerpio Pharmaceuticals: C; Alcon Surgical (RACII): C; Alkahest: C; Alimera Sciences: C; Allergan: C; Amgen: C; AMO: C; Annidis: C; ArticDx: C; Arctic Vision: C; AsclepiX Therapeutics: C; ASRS Board: C; Avalanche: C; Bausch + Lomb: C; Beyeonics: C; Boehringer Ingelheim: C; BridgeBio/Retinagenix: C; Carl Zeiss: C; CDR-Life: C; Chengdu Kanghong Biotechnology: C; Clearside Biomedical: C; Daiichi Sankyo: C; Dose Medical: C; Fox Kiser: C; Gemini Pharmaceuticals: C; Glaukos: C; Graybug Vision: C; Ionis: C; Irenix: C; Iveric bio: E; jCyte: C; Kodiak Sciences: C; Lutronic: C; Lux Biosciences: C; MacuSight: C; Merck: C; Nan Fung Group: C; NeoVista: C; Neurotech: C; Novartis/Alcon Pharmaceuticals: C; Oculis: C; Omeros: C; Ophthotech: C; Opthea: C; Optovue: C; Orbis International: C; Oxurion/ThromboGenics: C; Palatin Technologies: C; PanOptica: C; Pentavision: C; Pieris Pharmaceuticals: C; pSivida /Eyepoint Pharmaceuticals: C; Regenxbio: C; ReNeuron: C; Retina World Congress: C; Roche/Genentech: C; Santen: C; SciFluor Life Science: C; Shire Human Genetics: C; Spark Therapeutics: C; Stealth BioTherapeutics: C; Topcon: C; TrueVision: C; Verana/DigiSight: C.
Dr. Freund Allergan: C; Genentech/Roche: S; Novartis/Alcon Pharmaceuticals: C; Optovue: C; Heidelberg Engineering: C; Spark Therapeutics: C; Zeiss: C.
Dr. Lauer Allergan: S; Biogen: C; Genentech: S; NEI: S; Nightstar/Biogen: C; Oxford BioMedica: S; Regenxbio: C; Sanofi: C.
Dr. Yannuzzi None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|