Download PDF
Unless vision begins deteriorating, observation without treatment appears to be a safe and effective management strategy in patients with center-involved diabetic macular edema (CI-DME) and good baseline visual acuity (VA), a landmark study by the Diabetic Retinopathy Clinical Research network has concluded.1
Protocol V. The prospective, randomized clinical trial, called Protocol V, compared visual outcomes in 702 eyes with CI-DME that were managed initially with aflibercept (Eylea), laser photocoagulation, or observation. All study subjects began the trial with VA of at least 20/25, and aflibercept treatment was initiated in the observation eyes (and in the laser-treated group) if vision worsened during follow-up.
After two years, there was no significant difference in final mean VA among the three groups, the researchers reported. The percentage of eyes with at least a 5-letter VA decrease compared to baseline was 16% (33/205), 17% (36/212), and 19% (39/208) in the aflibercept, laser, and observation groups, respectively.
Furthermore, two-thirds of the observation eyes and three-quarters of the laser-treated eyes never required any intravitreal injections during the two-year period, said Carl W. Baker, MD, the research network’s Protocol V chair.
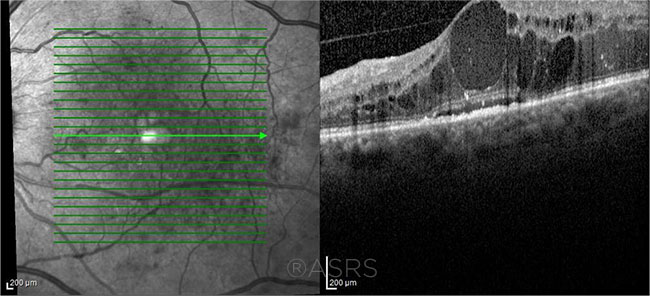 |
WATCHFUL WAITING? This patient’s DME (shown here at initial presentation) was treated with an anti-VEGF injection. Observation without treatment may be appropriate for selected eyes, results of Protocol V indicate. This image was originally published in the ASRS Retina Image Bank. Michelle V. Carle, MD, MMEd, and Ronnie Calip. Diabetic Macular Edema Initial Presentation. Retina Image Bank. 2018; Image Number 28496. © The American Society of Retina Specialists.
|
Support for watchful waiting. The evidence that a large subset of DME patients can be managed successfully with watchful waiting is important, because approximately 40% of DME eyes presenting to ophthalmologists have vision of 20/25 or better, said Dr. Baker, a vitreoretinal specialist who practices in Paducah, Kentucky.
“Vitreoretinal specialists see a lot of DME patients like these, but until this point we haven’t really known the best way to treat them,” he said. “Our study demonstrated that with these patients you could wait until vision drops and, with careful observation, you end up with the same chance for good results after two years.”
Areas of concern. In clinical practice, following the study’s findings could pose issues of compliance, Dr. Baker said, because patients under observation must unfailingly return for frequent examinations every eight to 16 weeks. “We don’t know how the issues of compliance might affect outcomes in these three different groups,” he noted.
Concerns also have been raised that the Protocol V findings could negatively impact decisions about reimbursement for intravitreal injections, Dr. Baker said. “It’s important for us to sit down with clinicians inside and outside our research network and discuss the implications of these results” as they relate to working with third-party payers, he said. “We certainly would not want this to limit the availability of treatment options for our patients.”
Dr. Baker said the results support a more personalized approach to interventions for DME. “This is another point we are going to make with the payers: that these strategies were shown to be successful but that we used the individual patient’s progress or loss of vision to determine whether they were going to get the injectable medicine. Each individual DME patient had different needs that had to be met,” he said.
Next steps. The Protocol V researchers, who practice at 91 sites in the United States and Canada, plan to conduct further analyses of study data to try to better define factors affecting DME progression. They also will look for biomarkers that might be used to identify patients most at risk, Dr. Baker said.
—Linda Roach
___________________________
For more on Protocol V, listen at aao.org/audio/episode-168-drcr-net-protocol-v-study-results-real. The two-part episode covers study design, results, and real-world implications.
___________________________
1 Baker CW et al., for the DRCR Retina Network. JAMA. Published online April 29, 2019.
___________________________
Relevant financial disclosures—Dr. Baker: Alcon: S; Genentech: S; Novartis: S; Regeneron: S.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Annabi NIH: S; UCLA: S; U.S. Department of Defense: S.
Dr. Baker Alcon: S; Genentech: S; Novartis: S; Regeneron: S.
Dr. Solomon Alcon: C,S; AqueSys: C; ClarVista Medical: C; Glaukos: C; Icon Bioscience: C; Integrity Digital Solutions: C; Mati Therapeutics: C; Octane Visionary VC Fund: C; OcuHub: C; Omeros: C; PogoTec: C; PRN: C; Versant Ventures: C.
Dr. Stewart Carl Zeiss Meditec: C; Genentech: C; Merck: C.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review