By Duncan Berry, MD, Pradeep Mettu, MD, and M. Tariq Bhatti, MD
Edited by Steven J. Gedde, MD
Download PDF
About three months ago, 78-year-old Betty Applewhite* got out of bed on a typical winter morning. As soon as her feet hit the floor, she could tell something wasn’t right. After taking a few steps, she was overcome by dizziness and had to brace herself on the nightstand. Gathering herself, she made her way to the kitchen, where she began to feel nauseated. Although she was recovering from a recent bout of sinusitis, these symptoms of dizziness and nausea were altogether different and more worrisome.
Ms. Applewhite called a friend who took her to the local emergency department, where it was evident that she was having serious balance problems. She was admitted so that a transient ischemic attack (TIA)/stroke could be ruled out. The results of the initial workup—including basic labs, non-contrast CT scan of the head, and MRI of the brain and orbits with contrast— were negative.
The neurology service was consulted. They agreed that Ms. Applewhite did have some gait instability, but they saw no evidence of acute stroke and recommended that she be discharged to a subacute rehabilitation facility. There, Ms. Applewhite complained to the staff of blurry vision. It was noted that she had bilateral conjunctival injection, and she was referred to our clinic for further evaluation.
 |
|
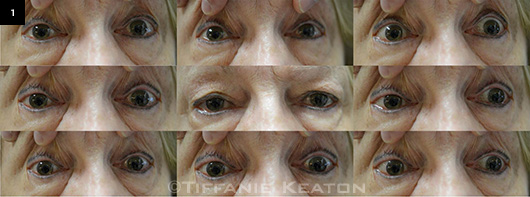
WE GET A LOOK. While many of the patient's test results were normal, her motility was limited in all directions.
|
We Get a Look
When we saw Ms. Applewhite, she reported that since her troubles began five days earlier, she had noticed that her vision was blurry. She did not feel that one eye was worse than the other. She also stated that both of her eyes felt “grainy”—as if she had dust or sand in them. In response to our questions, she reported no binocular diplopia, eye pain, itching, epiphora, or photosensitivity.
Her past ocular history was significant for cataract extraction and intraocular lens placement in her right eye six months prior and in her left eye five months prior. She said that these surgeries had not involved any complications.
Her medical history was significant only for hypertension and diabetes mellitus type 2, for which she was being treated with both metformin and insulin.
What We Found
On our exam, her best-corrected visual acuity (BCVA) was 20/25 in her right eye and 20/40-2 in her left eye. Her intraocular pressure was 15 mmHg bilaterally.
She had mild anisocoria, with the right pupil measuring 3.5 mm and the left pupil measuring 3 mm. The anisocoria was the same in the light or dark. Pupillary light reflexes were normal in each eye, with no relative afferent pupillary defect.
Her confrontation visual fields were full to counting fingers bilaterally, and she identified 10 of 10 Ishihara color plates bilaterally.
The external examination was remarkable only for very mild ptosis on the right with a marginal reflex distance-1 of 2.5 mm on the right and 3 mm on the left. She had normal levator function and no evidence of proptosis by Hertel exophthalmometry. Her orbicularis oculi muscle strength was normal on both sides.
Slit-lamp exam was notable only for mild diffuse conjunctival injection bilaterally and was otherwise within normal limits.
Her dilated fundus exam revealed healthy-appearing optic nerves in each eye with normal cup-to-disc ratios, without evidence of pallor or edema. The remainder of her fundus exam was notable for dot-blot hemorrhages in all four quadrants in each eye, but there was no evidence of neovascularization or other signs of proliferative diabetic retinopathy.
On motility testing, it quickly became apparent that Ms. Applewhite had severely limited movements of the eyes in all directions of gaze (Fig. 1).
Our Differential Diagnosis
The differential for bilateral external ophthalmoplegia is broad and includes a host of rarely encountered syndromes (see table below).1
In the context of our patient’s presentation, however, we were able to narrow it down to a select few. As is helpful with the evaluation of any motility problem, we thought about our differential in categories localizing to one of the following: the central or peripheral nervous system, the extraocular muscles, or the neuromuscular junction.
Central and peripheral nervous system. In a 78-year-old woman with balance problems and bilateral ophthalmoplegia, Wernicke encephalopathy was close to the top of our differential, even though the patient was not overtly encephalopathic and did not have a history of alcoholism or other risk factors for thiamine deficiency. Also, as we had only a self-reported medical history from the patient, we considered neurosyphilis as well.
Extraocular muscles. For myogenic causes, we thought it reasonable to consider both giant cell arteritis and thyroid eye disease. Additionally, given the patient’s history of type 2 diabetes requiring insulin, we certainly did not want to overlook an infectious etiology such as invasive mucormycosis, although the lack of other symptoms made the diagnosis unlikely.
Neuromuscular junction. Myasthenia gravis should always be on the differential for causes localizing to the neuromuscular junction. A more rare cause to consider in the setting of bilateral ophthalmoplegia and balance problems is Miller Fisher syndrome.
Making the Diagnosis
In clinic, we were able to obtain all of the patient’s records from her recent hospitalization. We confirmed that she did in fact have a thorough workup for her balance problems, including CT of the head, MRI of the brain and orbits with contrast, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), complete blood count (CBC), rapid plasma reagin (RPR), and lumbar puncture with cerebrospinal fluid (LP with CSF) analysis—all of which were normal. These findings essentially ruled out all of the etiologies on our differential except Wernicke encephalopathy, myasthenia gravis, and Miller Fisher syndrome.
We were also able to review the neurology consult, which noted in the physical exam section that “ … she does have a very slight ataxia with walking.” It also said, “Reflexes were hypoactive throughout. Ankle reflexes were present but again hypoactive. Plantar reflexes were silent.”
While these physical exam findings were not emphasized in the consult note, they were key to making the diagnosis in this patient. We now had a triad of findings—bilateral external ophthalmoplegia, ataxia, and areflexia—that fit the classic clinical description of Miller Fisher syndrome (MFS).
Discussion
The triad of bilateral ophthalmoplegia, ataxia, and areflexia was first described by Collier in 1932 as a variant of Guillain-Barré syndrome (GBS) and was eventually classified as a separate entity by C. Miller Fisher in 1956.2,3 It is reported to affect men twice as often as women. Mean age of onset is 43.6 years4.
Miller Fisher syndrome is primarily a clinical diagnosis; however, additional diagnostic measures can be taken. Specifically, the anti-GQ1b IgG antibody has been found to be present in 85 percent of patients with a clinical diagnosis of MFS.5 This specific test is usually part of a larger antiganglioside antibody panel that includes other antibodies associated with neuromuscular disorders. Importantly, the workup for MFS should also include neuroimaging and labs to rule out causes such as Wernicke encephalopathy, vascular brainstem disease, multiple sclerosis, myasthenia gravis, brainstem neoplasm, and a variety of bacterial or viral brainstem encephalitides.4
MFS is a self-limiting process, and most patients improve completely within 8 to 12 weeks without treatment. There have been no randomized controlled trials evaluating treatment modalities for MFS. Analysis of the largest MFS case series failed to show any beneficial effects in the patients who had received plasmaphoresis compared with the group that received no immunotherapy.6 That said, in patients with systemic findings consistent with acute inflammatory demyelinating polyneuropathy (also known as GBS), treatment with intravenous immunoglobulin, corticosteroids, and/or plasmaphoresis is indicated.
Follow-up
Despite the anti-GQ1b IgG being negative, we felt that Ms. Applewhite had MFS. We discussed the natural history of the disease with her and recommended observation. She was not able to follow up with us in clinic. However, in a telephone interview at one month, she said that her ataxia had resolved, but she complained of diplopia consistent with persistent ophthalmoplegia. In another telephone interview at three months, she reported that all of her symptoms had resolved, and she had normal clinical findings as assessed by both her primary ophthalmologist and neurologist.
___________________________
* Patient’s name is fictitious
___________________________
1 Sergott RC et al. Ophthalmology. 1984;91(1):18-22.
2 Collier J. Edinburg Med J. 1932;39:601-618.
3 Fisher M. N Engl J Med. 1956;255(2):57-65.
4 Berlit P et al. J Clin Neuroophthalmol. 1992;12(1):57-63.
5 Nishimoto Y et al. J Neuroimmunol. 2004;148(1-2):200-205.
6 Mori M et al. J Neurol Neurosurg Psychiatry. 2002;72(5):680.
___________________________
Dr. Berry is an ophthalmology resident, Dr. Mettu is a fellow in neuro-ophthalmology, and Dr. Bhatti is a professor of ophthalmology and neurology and chief of the neuro-ophthalmology service; all three are at the Duke University Eye Center. The authors report no related financial interests. Funding was provided by an unrestricted departmental grant from Research to Prevent Blindness.