Download PDF
Although cataract surgery is already one of the safest, most effective surgeries worldwide, its evolution continues. Three technologies—1 now in use and 2 in development—may go a long way toward transforming the field, whether by reducing the need for postoperative drops, revolutionizing the approach to intraocular lens (IOL) adjustment, or allowing the clinician to circumvent cataract surgery altogether.
 |
|
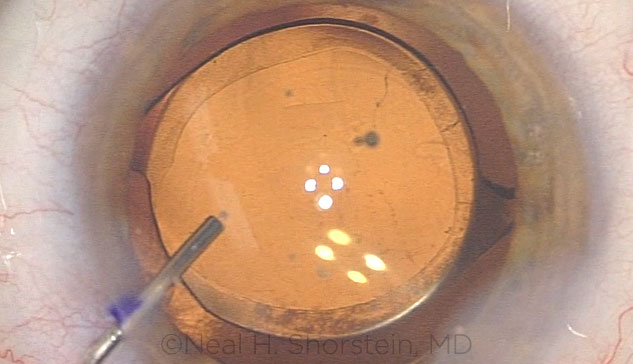
ANTIBIOTICS. Intracameral delivery of antibiotics immediately after cataract surgery. The procedure emerged in 2005 with the publication of a European study.
|
Moving Toward Drop-Free Surgery
Around the turn of the millennium, the incidence of endophthalmitis in the United States was reported to range from 1 in 300 to 1 in 1,000, said Neal H. Shorstein, MD, at the Kaiser Permanente Medical Center in Walnut Creek, California. “Today, it’s more typical to see rates of 1 in 5,000 to 1 in 10,000,” he said.
What’s behind this decrease? “One reason is that surgeons are more aware of wound construction and management,” Dr. Shorstein said. Another contributing factor may be the increasing adoption of intracameral antibiotics (IA)—that is, direct delivery to the inside of the eye right after cataract surgery.
Making inroads. Although IA is a controversial practice, it is gaining momentum, based in part on the following.
Drug delivery. With IA, you inject directly into the anterior chamber, where you want the drug to stay for some time after surgery, said Dr. Shorstein. “Depending upon the agent and how much you are injecting, the concentrations in the eye are on the order of about 1,000 to 3,000 micrograms per milliliter—high enough to overcome even resistant strains of coagulase-negative Staphylococcus, one of the most common causative organisms,” he said. “With topical drops applied to the surface of the eye, however, the concentration of antibiotic in the anterior chamber is too low to overcome organisms with higher resistance.”
Patient perspective. In general, IA is a financial win for patients because they will need fewer medications, said Michael Greenwood, MD, at Vance Thompson Vision in Fargo, North Dakota.
In addition, IA can circumvent problems with adherence. “Patients often have difficulty instilling eyedrops” and may inadvertently scratch their conjunctiva or cornea with the eyedrop container, Dr. Shorstein said. “Or they may never purchase their drops, fail to instill them in proper intervals, or simply stop using them (prematurely).” A quick, one-time injection by the surgeon circumvents these problems.
Lingering concerns. Those who are uneasy with the widespread adoption of IA cite the need for more level 1 evidence from randomized clinical trials (see “IA Research Notes”). Other barriers to its use include the following.
No FDA approval. U.S. surgeons do not have an FDA-approved IA agent available. Instead, ophthalmologists may have their hospital compound the antibiotic or use compounding pharmacies such as ImprimisRx, Leiters, and Avella Specialty Pharmacy, which are FDA-registered 503B outsourcing facilities, said Dr. Greenwood.
And because the surgery center generally bears the cost of procuring the product, this adds another barrier in terms of increased operating costs.
Potential toxicity. Surgeons may also worry about compounding errors, which can lead to insufficient antibiotic strength or toxicity, said Dr. Greenwood. For example, a primary risk of cefuroxime, said Dr. Shorstein, is temporary or permanent macular toxicity. In rabbit studies and in human tissue culture, moxifloxacin has displayed a risk of corneal endothelial toxicity. Another potentially blinding complication is hemorrhagic occlusive retinal vasculitis (HORV), which has been linked to the use of vancomycin (see “Questions about half-life,” below).
“Any time you’re using a compounding pharmacy, you want to make sure it is following federal regulations,” Dr. Greenwood said. Similarly, if your clinic or local hospital is doing the compounding, the process must be painstakingly accurate and in accordance with all regulations, he said.
Questions about half-life. The research in the literature is slightly divergent on the exact half-life of drugs in the anterior chamber, said Dr. Shorstein. “For cefuroxime and moxifloxacin, the concentration is above typical organisms’ MIC90 for about 4 to 6 hours. For vancomycin, it’s longer.” However, because of the risk of HORV, he noted, the FDA and the American Society of Cataract and Refractive Surgery (ASCRS) strongly advise against the routine injection of vancomycin for the prophylaxis of endophthalmitis.
Intracameral modifications. Many surgeons who use IA also combine it with another medication. In addition, some surgeons use IA without adding topical antibiotic drops, Dr. Shorstein said (see “Going drop free,” below).
Dr. Greenwood places Dex-Moxi-Ketor (ImprimisRx) into the anterior chamber after surgery. (Dex-Moxi-Ketor is short for dexamethasone, moxifloxacin, and ketorolac, which are steroid, antibiotic, and nonsteroidal anti-inflammatory medications, respectively.) He and his colleagues1 found that “intravitreal injection of an antibiotic and a steroid does not create significant intraocular pressure (IOP) spikes following cataract surgery in patients with glaucoma,” he said.
Dr. Greenwood’s approach is not entirely drop free, however. “For a month, patients take 1 drop once a day of a combination of prednisolone acetate, gatifloxacin, and bromfenac, a combination medication that is also from ImprimisRx,” he said. By doing so, he explained, he’s eliminated about 80 drops from his cataract patients’ postsurgical regimen.
Going drop free. In contrast, before cataract surgery, Dr. Shorstein’s patients receive only a dilating drop. After cataract surgery, he has patients apply no drops, pointing out that large studies have underscored the lower infection rates using IA alone (see “IA Research Notes”).
“Our study2 showed that an injection of triamcinolone, delivered subconjunctivally, is just as effective in preventing postoperative macular edema as topical postop steroid drops,” said Dr. Shorstein, who does prescribe topical steroid drops postoperatively for patients with glaucoma and a compromised optic nerve. “This long-acting steroid injection, along with the intracameral antibiotic injection, make up the drop-free technique.”
Other ways to lower risk. A variety of techniques further lower the risk of endophthalmitis, said Dr. Greenwood. This includes a good Betadine prep prior to surgery, sterile techniques during surgery, and placement of a Betadine solution on the eye at the end of surgery.
In addition, some studies have concluded that, because IOP can dip soon after surgery, it’s advantageous to perform stromal hydration at the end of the surgery. “Leaving the eye adequately pressurized with a slightly increased IOP helps seal the corneal flaps together and ensure wound closure following the procedure,” said Dr. Shorstein.
After surgery, he also instructs his patients to avoid touching or rubbing their eyes for 24 to 48 hours, and to not apply any artificial tears. “Although there’s no hard evidence, it’s my belief that the less patients manipulate their eyes, the lower the risk of endophthalmitis,” he said.
 |
|
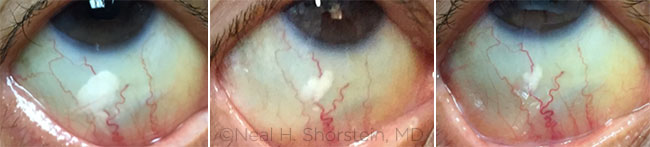
NO MORE DROPS? From left: One day, 4 weeks, and 6 weeks following a subconjunctival injection of 3 mg of triamcinolone acetonide. Some research indicates that this prevents postoperative macular edema as effectively as postop steroid drops do.
|
Rethinking Refractive Error Correction
Refractive index shaping—also known as refractive indexing—uses a minimally invasive, ultrafast femtosecond laser to change the refractive index ab interno of an IOL without measurably changing its shape, said Scott M. MacRae, MD, at the University of Rochester in New York. “The laser has about 100 times less pulse energy” than commercial femtosecond lasers now in use, he said.
Procedure basics. Before the laser adjustment, the subject receives topical anesthesia and drops to dilate pupils, said Liliana Werner, MD, PhD, at the University of Utah in Salt Lake City. The subject’s eye is aligned and docked to the femtosecond laser system, and appropriate laser treatment is then applied.
Research. Currently, 2 companies are evaluating the technology: Clerio Vision is working with researchers at the University of Rochester, and Perfect Lens is collaborating with researchers at the University of Utah.
“We are experimenting with different types of IOL materials to observe how they react and to determine the best energy levels to use,” said Dr. MacRae. “Although certain materials do change more than others, the response is very uniform for each type of material.” He added, “We have tested a variety of commercially available lenses and some noncommercial materials, and they have a predictable response to refractive indexing.”
The technology has not been tested with silicone or PMMA lenses, but it works well with commercially available hydrophobic and hydrophilic acrylic lenses, said Dr. Werner.
One option would be to use these types of monofocal lenses with the initial power selected for each eye, according to current standards of care, with the idea that they could be modified later. Another approach would involve developing a material that is very responsive to refractive indexing, which could provide even more control and flexibility, said Dr. MacRae.
Multiple adjustments possible? This technology opens up the possibility of responding to refractive error changes that occur over time, said Dr. MacRae. Many adjustments may be possible, said Dr. Werner, because each treatment is applied to only a very thin layer within the IOL. “Ongoing studies are assessing this, as well as the amount of power change that can be obtained before the quality of the IOL optic decreases,” she said.
Potential benefits. The laser treatment can be done in a noninvasive manner under topical anesthesia, and it is very fast, said Dr. Werner. “In our rabbit studies, the treatment took 23 seconds for a change of +3.6 D.” Other potential advantages include the following:
Precision. The precision obtained with the power adjustment by the femtosecond laser is within 0.1 D of the target and is very consistent, said Dr. Werner.
Address a wide range of refractive errors. “We know we can treat ± 4 diopters, and potentially quite a bit more, depending upon the type of [IOL] material,” said Dr. MacRae. He added that refractive indexing can be used to treat residual myopia, hyperopia, astigmatism, and higher-order aberrations, as well as to create diffractive bifocals, trifocals, and other patterns.
Flexibility. Analyses of the optical quality through modulation transfer function (MTF) measurements of the lenses3 show that a monofocal lens can be changed into a multifocal lens, with resulting MTF values for far and near foci similar to commercially available multifocal lenses, said Dr. Werner. (The MTF of an IOL is a measurement of its ability to reproduce the image of an object.)
“That same lens can be turned back into a monofocal lens, and the final MTF obtained is very close to the original MTF of the initial monofocal lens. This means that all of those changes can be performed without any significant decrease in the optical quality of the original lens.”
Negligible toxicity. Standard tests have been performed on modified IOLs, and no leachables were found, said Dr. Werner. Also, in vivo studies performed in rabbits showed no inflammatory reaction or signs of toxicity up to 6 months postoperatively. Anti-inflammatory treatment was not applied to the rabbit eyes after laser treatment, she added.4
Candidates. Who might eventually benefit from refractive indexing? One example is children with congenital cataracts, whose refractive error changes over time as the eye develops. Other potential recipients include those patients who have residual refractive errors after cataract surgery, who would like to have their monofocal IOL converted to a multifocal IOL, or who cannot adapt to their current multifocal IOL, said Dr. Werner.
Next up: results in humans. Dr. Werner expects results from the first human trial shortly. And more human data on refractive indexing will become available over the next 1-2 years, said Dr. MacRae. “Big issues that need to be fully worked out are reproducibility, long-term biocompatibility, and optical performance. All the work thus far has been done in animals or on the bench. It’s exciting, but we need to make the airplane fly.”
Other uses for refractive index shaping. Refractive index shaping also shows promise in 2 additional areas, Dr. MacRae said.
Modifying contacts. “Making diffractive and refractive index changes internally, rather than on the outside of the lens, means that [the clinician] could create a much thinner lens for high myopes or hyperopes, thus improving the oxygen permeability and comfort level,” he said. “You could also create a diffractive multifocal optic internally in the contact lens.” Doing so isn’t possible with the current generation of multifocal contact lenses, which essentially are designed as “refractive” multifocals, he said.
Treating corneas. If it becomes possible to use refractive index shaping on the cornea, that would be a game changer, said Dr. MacRae, as it does not significantly affect corneal nerves or provoke much in the way of a wound healing response. “If refractive indexing can treat higher degrees of refractive error, it has the potential to revolutionize the field,” he said. “And if the technology could be made portable, it could go a long way toward attacking the problem of refractive error.”
In addition, any cornea treatments can be placed inlayers so that multiple treatments could be done sequentially, as the refraction changes. “You can put a treatment in and go 20 microns deeper and then repeat the treatment, if needed,” he said. “For example, if you treat a 16-year-old with a diopter of myopia, you can re-treat at age 22 or 23, if she gains another diopter of myopia.”
Dr. MacRae explained that the energy levels used in refractive indexing are so low as to be nondisruptive. “We are micromachining the cornea and causing densification of the collagen fiber spacing, based on our histopathologic studies.” Animal models have demonstrated that this technology does work and is stable and persistent for at least 2 years, he said. With regard to impact upon keratocytes, the animal studies have found minimal, localized keratocyte death only within the laser focal zone.5
 |
|
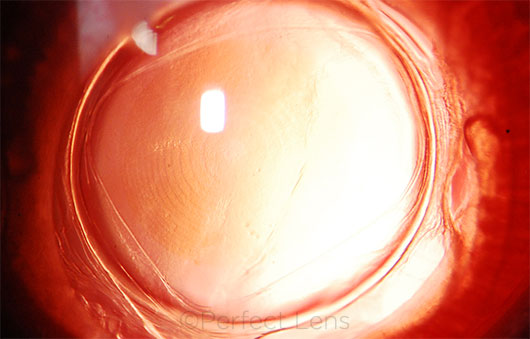
REFRACTIVE INDEXING. This image is of a rabbit eye implanted with a commercially available IOL, 5 hours after refractive index shaping. What looks like a multifocal pattern—visible individual zones in the IOL—is a refractive change.
|
Reducing Cataracts With an Eye Drop
In 2015, a team of researchers at the University of California, San Diego was reviewing the genetic makeup of 2 families with congenital cataracts. What they found was like so many serendipitous discoveries in medicine: Each family member with cataracts had a mutation in the lanosterol synthase gene (LSS), which had no previously known association with cataracts. This mutation stopped production of lanosterol, a naturally occurring steroid. “That led us to the idea that lanosterol was, in fact, important for keeping lens proteins from aggregating and producing cataracts,” said Kang Zhang, MD, PhD, who heads the research team.
Lanosterol. “We conducted studies using a naturally occurring age-related cataract in rabbits and dogs,” said Dr. Zhang.6 “We took the rabbits’ cataractous lenses out and incubated them in test tubes with lanosterol, showing that we could reduce cataracts and improve the clarity of the lens.”
His team then did an experiment in live dogs. “After 6 weeks of treatment using lanosterol eye drops, we were also able to reduce cataracts and significantly increase lens clarity.” Although 6 weeks was sufficient to reduce cataracts, he said, cataracts may recur, so retreatment may be needed. An eyedrop treatment has now been developed for treating cataracts in animals.
New nanoparticle. Now, Dr. Zhang and his team are turning their attention to developing a lanosterol eyedrop for humans. The biggest stumbling block has been the molecule itself, which is large and not easily soluble.
“But we have found a nice nanoparticle vehicle and developed a formula that can be used for delivery,” he said, explaining that the nanoparticle facilitates lanosterol crossing the cornea by creating an amphipathic molecule, which has both hydrophilic and hydrophobic parts. The lanosterol formula can be delivered as either an eye drop or by implantation, he said, and he added that his team “implanted this nanoparticle gel into the subconjunctival space in monkeys and found it can perform sustained delivery for 2-3 months.”
VP1-001. Another research team, at ViewPoint Therapeutics in San Francisco, is working on a second eyedrop. In a study published in Science, they reported on a compound that stabilized lens crystallin proteins and prevented them from forming amyloids. The compound, now named VP1-001, improved lens transparency in murine models of hereditary cataract.7 It also showed promise in aged mouse and human lenses.
Next up: Studies in humans. “In the last quarter of this year, we are going to initiate both human trials and animal studies in the United States and China,” said Dr. Zhang. He plans to enroll between 30 and 50 people in the phase 1 safety study, but expects toxicity to be minimal given the endogenous nature of lanosterol.
Looking ahead. Although cataract-dissolving eyedrops are unlikely to be used for rock-hard cataracts, Dr. Zhang sees this approach as a promising alternative to surgery in other instances—for example, with patients who are at risk of complications because they have certain eye conditions (such as weak zonules), bleeding disorders, and/or cardiovascular conditions.
The drops also might be appropriate for those patients who are bothered by symptoms such as glare or trouble seeing in dim light, but their symptoms are not considered severe enough to justify cataract surgery. Finally, eye drops could be widely distributed in remote, resource-scarce areas where surgery is difficult to deliver or even unavailable—and where the burden of cataract-related blindness is greatest.
___________________________
1 Kindle T et al. J Cataract Refract Surg. 2018;44(1):56-62.
2 Shorstein NH et al. Ophthalmology. 2015;122(12):2450-2456.
3 Nguyen J et al. J Cataract Refract Surg. 2018;44(2):226-230.
4 Werner L et al. J Cataract Refract Surg. 2017;43(8):1100-1106.
5 Wozniak KT et al. Exp Eye Res. 2017;165:20-28.
6 Zhao L et al. Nature. 2015;523(7562):607-611.
7 Makley LN et al. Science. 2015;350(6261):674-677.
 |
|
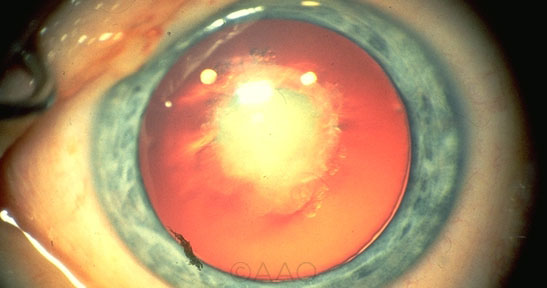
DISSOLVING CATARACTS? Research on drops to dissolve cataracts began with a fortuitous discovery linking congential cataracts (shown here) with mutations in the LSS gene.
|
IA Research Notes
Intracameral antibiotics entered the spotlight when a study by the European Society of Cataract and Refractive Surgeons (ESCRS) found that the rate of endophthalmitis was 5 times higher in those who did not receive an IA injection.1 “The results also showed that there was no statistical benefit in adding perioperative topical antibiotics along with intracameral antibiotics,” said Dr. Shorstein.
The study has received its fair share of criticism over the years, however, particularly with regard to its design,2,3 and additional randomized clinical trials are needed.
Kaiser study. Dr. Shorstein and his colleagues4 found the following in a study of 300,000 surgeries:
- IA reduced the incidence of endophthalmitis by about half, with no measurable differences between cefuroxime and moxifloxacin.
- Adding topical antibiotics to a regimen of IA did not further reduce the risk of endophthalmitis. In fact, doing so actually increased the risk of endophthalmitis, a finding that was not statistically significant. Dr. Shorstein suspects that any increase could be due to bottle tip contamination, patient error in administration, or trauma to the eye from applying drops.
- Patients on topical fluoroquinolone or polymyxin/trimethoprim alone experienced a significantly lower incidence of endophthalmitis compared to those who failed to fill their prescription for drops and to those on a topical aminoglycoside.
Up next. An ASCRS study is set to compare topical and intracameral moxifloxacin. The hope is that the investigation will lead to FDA approval of an intracameral indication for this existing antibiotic drug, Dr. Greenwood said.
___________________________
1 Endophthalmitis Study Group. J Cataract Refract Surg. 2007;33(6):978-988.
2 Schwartz SG et al. Ophthalmology. 2016;123(7):1411-1413.
3 George NK, Stewart MW. Ophthalmol Ther. Published online July 5, 2018.
4 Herrinton LJ et al. Ophthalmology. 2016;123(2):287-294.
|
Meet the Experts
Michael Greenwood, MD Ophthalmologist at Vance Thompson Vision in Fargo, N.D. Relevant financial disclosures: None.
Scott M. MacRae, MD Director of refractive services in the department of ophthalmology at the University of Rochester and professor of ophthalmology and visual science at the University of Rochester’s Center for Visual Science in Rochester, N.Y. Relevant financial disclosures: None.
Neal H. Shorstein, MD Ophthalmologist and associate chief of quality at the Kaiser Permanente Walnut Creek Medical Center in Walnut Creek, Calif. Relevant financial disclosures: None.
Liliana Werner, MD, PhD Professor of ophthalmology and visual sciences and codirector of the Intermountain Ocular Research Center at the John A. Moran Eye Center at the University of Utah in Salt Lake City. Relevant financial disclosures: Perfect Lens: S.
Kang Zhang, MD, PhD Professor of ophthalmology, chief of ophthalmic genetics; founding director, Institute for Genomic Medicine; and codirector of biomaterials and tissue engineering, Institute for Engineering in Medicine, University of California, San Diego. Relevant financial disclosures: None.
Full Financial Disclosures
Michael Greenwood, MD Alcon: L; Equinox: C,O; Glaukos: L; New World Medical: C,L; Staar: L.
Scott M. MacRae, MD None.
Neal H. Shorstein, MD None.
Liliana Werner, MD, PhD Abbott Medical Optics: S; Advanced Vision Science: S; Alcon: C,S; Anew Optics: S; Carl Zeiss Meditec: S; CIMA Technology: S; ClarVista Medical: S; Clearsight: S; Genisphere: S; Hoya: S; LensGen: S; Medennium: S; Merck: S; Omega: S; Perfect Lens: S; Physiol: S; PowerVision: C; Refocus Group: S; Shifamed/Atia: S.
Kang Zhang, MD, PhD None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|