Download PDF
Cataract surgery may be performed safely in patients who have survived infection with the Ebola virus and who test negative for the virus in ocular fluid specimens.1 This finding, from the EVICT (Ebola Virus Persistence in Ocular Tissues and Fluids) study, could potentially affect thousands of West Africans who are Ebola virus disease (EVD) survivors and are now at risk for ocular complications that may require surgery.
“Following their acute illness, EVD survivors in West Africa remain at very high risk for uveitis, which can lead to blindness and cataract,” said lead author Jessica G. Shantha, MD, at the Emory Eye Center in Atlanta. Uveitis has been estimated to affect 13% to 34% of EVD survivors.1
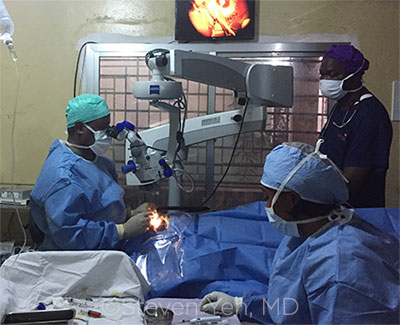 |
EBOLA SURGERY. Moges Teshome, MD, from Christian Blind Mission International, performs cataract surgery with the assistance of Johnny Sawyer and Hannah Dowie.
|
EVICT. This study is the first to evaluate the persistence of the Ebola virus in the eyes of EVD survivors with cataract or active inflammation. The stepwise approach employed in this cross-sectional study involved ocular screening, ocular fluid sampling, and subsequent manual small-incision cataract surgery in selected patients.
All told, 137 EVD survivors were screened, and 50 were enrolled. All tested negative for Ebola at 2 time points. Study findings include the following:
- Of the 50 patients in the study, 46 (92%) had visually significant cataract and a history of uveitis, and 2 (4%) had active uveitis.
- Thirty-four patients (34 eyes) underwent cataract surgery (surgery was deferred in the remaining 12).
- Postoperative visual acuity (VA) improved by ≥3 lines in 27 of the 34 patients, with 20 (59%) achieving a postoperative VA of ≥20/40.
The VA of 5 patients remained poorer than counting fingers due to vitreoretinal pathology.
Lessons learned. “We feel confident that cataract surgery can be performed safely with vision restorative outcomes at the time points assessed in our study,” said coauthor Steven Yeh, MD, also at Emory. “However, strict infection control precautions are recommended.” (For instance, in this study, eye care providers performed the ocular fluid sampling procedure while wearing full personal protective equipment.)
Looking ahead. Dr. Yeh stressed the need for formal consensus guidelines regarding timing of surgery and necessary surgical precautions. He also noted that more research is needed about the potential for Ebola to remain in ocular fluids and tissues.
The study does offer lessons about patients with uveitis syndromes related to other pathogens, such as herpes simplex virus or the Zika virus, Dr. Yeh noted. For instance, operating on inflamed eyes in patients with infectious uveitis should be avoided.
As for EVD, he said, “There is currently no known risk of Ebola virus transmission through casual contact, including the eye exam of a survivor. Strict hand-washing precautions and clinic sterilization strategies are recommended for medical care of EVD survivors.”
—Miriam Karmel
___________________________
1 Shantha JG et al. EBioMedicine. 2018;30:217-224.
___________________________
Relevant financial disclosures—Dr. Shantha: Santen; C. Dr. Yeh: Alcon: S; Clearside Biomedical: C; Santen: C.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Chang Carl Zeiss: C; Eyenovia: O; Iantech: C,O; Icon Bioscience: O; iDrops: C,O; Ivantis: C,O; Johnson & Johnson Vision: C; Mynosys: O,C; PowerVision: C,O; Presbyopia Therapies: O; RxSight: O,C; Slack: P; Versant Ventures: O.
Dr. Holekamp Alimera Sciences: C,L,S; Allergan: C,L,S; BioTime: C; Genentech: C,L,S; Katalyst: C,P; NotalVision: S; Novartis: C; Ophthotech: S; Ohr Pharmaceutical: S; Regeneron: C,L.
Dr. Shantha Santen: C.
Dr. Yeh Alcon: S; Clearside Biomedical: C; Santen: C.
Dr. Yin None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review