By Linda Roach, Contributing Writer, interviewing Emily Y. Chew, MD, Michael J. Elman, MD, George Mavro, and George E. Sanborn, MD
Download PDF
For patients who have lost vision in one eye to wet age-related macular degeneration (AMD), preserving functional acuity in the fellow eye is paramount. Yet clinical experience and research have shown that these and other high-risk patients often fail to use the Amsler grid between office visits to catch the earliest signs of neovascularization in their good eye.1
For the first time, there is a readily available solution to this ongoing dilemma: at-home hyperacuity monitoring for these and other high-risk eyes.
Detecting Early Onset
“One of the biggest predictors of how you’re going to do 2 years after your first anti-VEGF injection is how good your vision was to start off with,” said Emily Y. Chew, MD, with the National Eye Institute (NEI). “This new type of home monitoring is very important because it helps us identify AMD patients at the early onset of the neovascularization [process], when their baseline vision is good, maybe as good as 20/20 or 20/30, and the greatest amount of functional vision can be preserved.”
 |
|
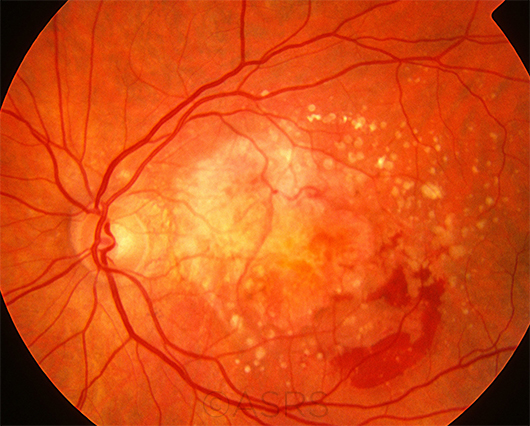
WET AMD. The goal of at-home monitoring is to catch the earliest signs of neovascularization and avoid problems such as those seen in this patient, who has multiple drusen, scar tissue, and hemorrhages due to active CNV.
This image was originally published in the ASRS Retina Image Bank. Henry J. Kaplan, MD, and Niloofar Piri, MD. Age-related Macular Degernation. Retina Image Bank. 2013; Image Number 5471. © The American Society of Retina Specialists.
|
Comparing Options
Two prescription-only at-home monitoring systems are now available in the United States. They differ in their assessment methodology and in the level of evidence for their clinical efficacy.
ForeseeHome. The ForeseeHome AMD Monitoring System (Notal Vision) proved its efficacy in a large controlled clinical trial sponsored by the NEI,2 and commercialization of the system began in spring of 2016.
How it works. The system consists of a preferential hyperacuity perimetry (PHP) device, plus proprietary metamorphopsia-detection algorithms and the specific telemonitoring protocols followed at a central reading center. Notal Vision provides the equipment to patients after receiving a prescription from an ophthalmologist.
When an AMD patient performs a test—ideally, this is done daily—the results are transmitted electronically to the reading center for computerized scrutiny. If the machine and the algorithm find suspicious test results, manual comparison to the patient’s baseline values is done by a specially trained ophthalmologist or optometrist, said George Sanborn, MD, with ForeseeHome. He added that he also personally sees all alerts and abnormal results. “And I look at trends once a week for patients who we think might have problems down the road.”
With regard to test results, the location and size of metamorphopsia and scotomas are derived from the patient’s response patterns to a series of dotted lines that the perimetry device flashes at various locations in the visual field. Some of the lines are straight; others have small deviations that the patient acknowledges by clicking a mouse. When compared with the patient’s baseline tests, errant or missing clicks are suggestive of metamorphopsia that might be due to early choroidal neovascularization (CNV).
Patients receive automatic reminders if they neglect to test themselves regularly. The treating ophthalmologist receives longitudinal monthly reports, as well as immediate notice of abnormal readings, so that an examination can be scheduled promptly.
Evidence of efficacy. For more than a decade, the PHP system has undergone increasingly rigorous tests of its technical and clinical performance. A critical moment came in April 2013, with interim analysis of data from the AREDS2-HOME (Age-Related Eye Disease Study 2-Home Monitoring of the Eye) study.2
For this prospective controlled clinical trial, 763 patients were randomized to device monitoring, while 757 were randomized to standard care and followed for a mean of 1.4 years. At the preplanned interim analysis, 51 of those in the device arm were detected as having progressed to CNV, compared with 31 in the standard care arm. Moreover, VA was significantly better at the time of CNV detection in the device group: 87% of patients in that arm had 20/40 or better, compared with 62% in the standard care arm. These findings led to the study’s early termination.2
“In my more than 30 years of doing large-scale research, this is the only trial that I know of where an independent data safety monitoring committee stopped the study early because the intervention worked so well,” said Michael J. Elman, MD, a coinvestigator in the trial. “The committee thought that the results were not going to change if we continued the study—and that withholding the system from the patients who were not receiving the intervention would be unethical.” In his own practice, Dr. Elman now has more than 120 patients using the system.
Cost. Early this year, federal officials approved the ForeseeHome reading center as an “independent diagnostic testing facility” covered by Medicare. The patient’s Medicare copay for the service is about $15 a month, with no patient costs for the equipment and setup. (There is also no cost to the prescribing clinician, and the device is shipped directly to the patient’s home.)
myVisionTrack. The smartphone- and tablet-based myVisionTrack (mVT) app (Vital Art and Science) is intended to look for signs of progression in eyes with existing maculopathies, including wet and dry AMD, diabetic retinopathy, and diabetic macular edema. Under the U.S. Food and Drug Administration’s 510K process, the mVT app has been cleared for U.S. marketing based on its equivalence to the Amsler grid and ForeseeHome.
How it works. The mVT test’s goal is to detect signs of macular metamorphopsia by displaying a series of images of 4 circles, one of which has an irregular edge, on a smartphone or tablet screen. The degree of distortion decreases with each subsequent image. The patient uses the touchscreen on the smartphone/tablet (either Apple or Android) to choose the distorted shape, and the results are tracked remotely. Performance is compared with the patient’s baseline values, and the prescribing doctor is alerted to abnormal test results.
Evidence of efficacy. So far, the published literature about mVT’s clinical performance has consisted largely of small validation studies,3 case series, and a prospective 16-week study designed to assess the ease of use and feasibility of monitoring macular changes with the smartphone app.4 In the latter study, 84.7% of the 160 patients adhered to daily mVT testing, and 98.9% performed at least weekly mVT testing.4
There is no Level I evidence from a large randomized controlled clinical trial comparing visual outcomes with mVT monitoring to outcomes achieved with standard care. However, the company believes that the existing limited studies and anecdotal reports, when combined with what was learned in AREDS2-HOME, support use of the mVT app to improve patient care, said George Mavro, with Vital Art and Science.
Cost. The patient costs for use of the mVT system have not been decided yet, nor has insurance coverage been approved. “We anticipate offering reimbursement in the next 12 to 24 months for both AMD and diabetic retinopathy patients,” said Mr. Mavro.
Differences to Consider
Target population. Medicare’s coverage criteria specify that ForeseeHome is appropriate only for AMD patients who are at high risk for CNV because of the presence of bilateral large drusen, or who have preexisting wet AMD in one eye, Dr. Sanborn said. The mVT app can be prescribed to monitor any type of maculopathy, Mr. Mavro said.
Initial visual acuity. Eyes that are to be monitored with ForeseeHome should have an initial VA of 20/60 or better. The recommendation for mVT is 20/100 or better.
Field of view. The mVT system does macular testing, and it covers a 3-degree center section of the central visual field. ForeseeHome looks for metamorphopsia both centrally and outward to the retinal periphery, covering 14 degrees of the central visual field.
Ease of use. In the feasibility trial of the mVT app, no patients failed their screening because of inability to perform the test.4 In contrast, the AREDS2-HOME study found that about 20% of patients screened could not use ForeseeHome successfully, either because preexisting significant visual field defects interfered with the PHP test or because the patient could not adapt to the testing device, Dr. Elman said. Some of these failures occurred because the patient could not fixate on a center light, as required by the ForeseeHome system, he added. (Fixation is not necessary to use the mVT app.)
___________________________
1 Ying GS et al. Ophthalmology. 2012;120(1):122-129.
2 Chew EY et al.; AREDS2-HOME Study Research Group. Ophthalmology. 2014;121(2):535-544.
3 Wang YZ et al. Invest Ophthalmol Vis Sci. 2013;54(8):5497-5505.
4 Kaiser PK et al. Retina. 2013;33(9):1863-1870.
___________________________
Dr. Chew is deputy director of the Division of Epidemiology and Clinical Applications and chair of the AREDS2 studies at the NEI. Relevant financial disclosures: None.
Dr. Elman practices with the Elman Retina Group and is assistant professor of ophthalmology at the Johns Hopkins University School of Medicine in Baltimore. He also is chairman of the Diabetic Retinopathy Clinical Research Network (DRCR.net) Protocol I. Relevant financial disclosures: Notal Vision, C,L,O,S.
Mr. Mavro is chief marketing officer at Vital Art and Science in Richardson, Texas. Relevant financial disclosures: Vital Art and Science: E.
Dr. Sanborn is medical director of the Foresee-Home AMD Monitoring System. Relevant financial disclosures: Notal Vision: E.
For full disclosures and the disclosure key, see below.