Download PDF
A team of researchers from Mount Sinai in New York City is the first to demonstrate a cellular defect in exfoliation syndrome (XFS)—an age-related systemic disorder that leads to a disorderly buildup of protein aggregates in multiple organs. Among its many pathologic effects, XFS is linked with hearing loss, as well as cardiovascular and cerebrovascular disease. For ophthalmologists, the most familiar manifestation is exfoliation glaucoma, in which the material clogs the trabecular meshwork and hinders outflow.1 XFS has also been linked with increased risks of cataract and retinal vein occlusion.
Broadening the field of XFS study. The team delving into this poorly understood disorder includes Audrey Bernstein, PhD, associate professor of ophthalmology and cell biology, and J. Mario Wolosin, PhD, professor of ophthalmology—both at Icahn School of Medicine at Mount Sinai—collaborating with Robert Ritch, MD, the Shelley and Steven Einhorn Distinguished Chair in Ophthalmology, at New York Eye and Ear Infirmary. They are taking a cell biology approach to uncover the mechanisms of XFS.
“In recent years, XFS researchers have focused their study on a gene called lysyl oxidase–like 1 (LOXL1), which cross-links elastin in the eye,” said Dr. Bernstein. “XFS develops almost exclusively when the LOXL1 genotype includes certain single amino acid LOXL1 variants. However, these variants are also found in many people without the disease,” said Dr. Bernstein, “which means that LOXL1 is only a part of the story.”
For this reason, Bernstein and colleagues turned their attention to what unites all age-related diseases such as XFS: a problem with proteolysis and autophagy. Normally, certain cells degrade unnecessary or toxic cellular components in a process somewhat similar to the recycling role of sharks and buzzards in the natural world.
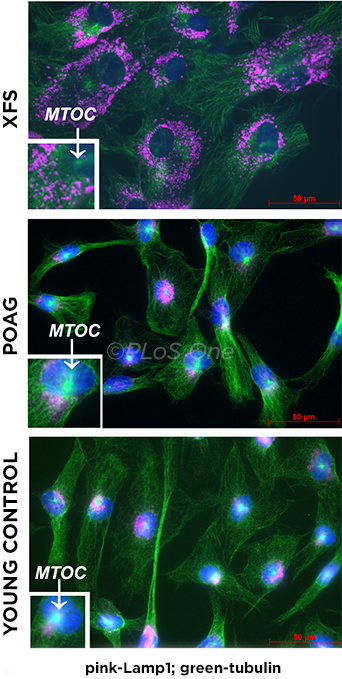 |
CELLULAR IMPAIRMENT. Lysosomes in XFS–tenon fibroblasts (TFs) cannot relocate to the perinuclear area under starvation conditions. TFs were seeded on collagen in a serum-free medium and immunostained for lysosomal-associated membrane protein 1 (Lamp1; pink) and b-tubulin (green). MTOC (microtubule organizing center), which organizes vesicular traffic, is absent in XFS. Bar = 50 μm.
|
Major findings. The researchers grew and compared cells from tissue samples normally discarded during surgery from patients with XFS, primary open-angle glaucoma (POAG), and strabismus. “We found that lysosomal function and autophagy were significantly dysfunctional in the exfoliation cells,” said Dr. Bernstein.
The exfoliation cells showed the following key characteristics: They were much larger than normal cells, proliferated slowly, and contained many disorganized vesicles. Under “starvation conditions” induced by removal of serum, the lysosomes and many vesicles in XFS cells did not relocate to the correct area and, therefore, failed to carry out their function of clearing cellular waste. In addition, a buildup of dead mitochondria—thought to cause oxidative stress—was likely due to an autophagy defect, said Dr. Bernstein.
Links with neurodegeneration research. “Our findings suggest that XFS is similar to diseases such as Alzheimer’s and Parkinson’s,” said Dr. Bernstein. “This is exciting because we can leverage what has been learned from thousands of studies conducted in the field of neurodegeneration.” In addition, she said, the accessibility of the eye provides a unique opportunity for testing therapies to reverse XFS pathology.
Ongoing research. Drs. Bernstein and Wolosin are now investigating the mechanisms underlying the autophagy failure. They are currently exploring whether there is a problem with LOXL1 folding that might directly cause XFS pathology. Further, is LOXL1 secreted into the extracellular space because waste-degrading processes inside the cell are dysfunctional?
Although these and other questions remain, Dr. Bernstein said, the findings from this research provide a basis for studying the mechanisms of this puzzling degenerative disease and, further, may suggest possible therapeutic approaches.
—Annie Stuart
___________________________
1 Want A et al. PLoS One. 2016;11(7):e0157404. doi:10.1371/journal.pone.0157404.
___________________________
Relevant financial disclosures—Dr. Bernstein: Bright Focus Foundation: S; Glaucoma Foundation: S; Research to Prevent Blindness: S; The MYS Family U.S. Charitable Foundation: S.
For full disclosures and disclosure key, see below.
More from this month’s News in Review