Download PDF
The antimetabolite drugs methotrexate (MTX) and mycophenolate mofetil (MMF) are commonly used as corticosteroid-sparing treatments for uveitic macular edema. But how do these treatments compare—and are they adequately effective? A clinical trial and subanalysis led by researchers at the University of California, San Francisco (UCSF) may help to answer these questions.
The head-to-head FAST (First-line Antimetabolites as Steroid-sparing Treatment) study found MTX and MMF to be similarly effective in the treatment of uveitis and uveitic macular edema.1 However, a recent subanalysis of FAST data has found that about half of all patients still had macular edema after 12 months.2
“We found comparable improvement and resolution of macular edema and improvement in visual acuity in both treatment groups, but approximately half of the patients had persistent edema after treatment,” said Nisha R. Acharya, MD, MS, at UCSF.
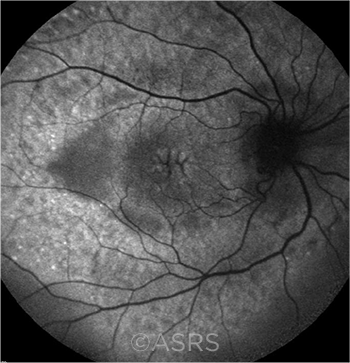 |
PERSISTENT. Macular edema in the right eye of a 17-year-old boy with chronic intermediate uveitis. This image was originally published in the ASRS Retina Image Bank. Hamid Ahmadieh, MD. Cystoid Macular Edema (CME). 2012; Image Number 701. © The American Society of Retina Specialists.
|
FAST basics. The multicenter FAST study was conducted to compare the efficacy of MTX versus MMF in uveitis. The researchers enrolled 216 patients who had noninfectious intermediate uveitis, posterior uveitis, or panuveitis. Patients were randomized to receive 25 mg of MTX weekly or 1.5 g of MMF twice daily for 12 months. A corticosteroid taper also was used.
All patients were assessed regularly using OCT and clinical exams. At six months, patients who achieved treatment success continued the same treatment, and those who failed treatment were switched to the other antimetabolite.
Subanalysis: A troubling surprise. At 12 months, median macular thickness in patients who stayed on the same treatment decreased from baseline by 23 μm in those treated with MTX and 18 μm in those who received MMF (p = .76). Resolution of macular edema was observed in 37% of eyes in the MTX group, versus 60% of eyes in the MMF group at 12 months (p = .10). Of those who switched treatments after six months, 47% of eyes on MTX and 55% of eyes on MMF had improvement of their macular edema at 12 months (p = .92).
“I expected both treatments would lead to improvement in macular edema and vision, and we found that. However, the finding that approximately 50% of patients had persistent edema was striking,” Dr. Acharya noted.
Bottom line. Dr. Acharya emphasized the importance of treating macular edema effectively, as it is a frequent cause of vision loss in uveitis patients. And the FAST results, as well as those from other research, suggest that “we may need to escalate or use additional adjunctive treatments to fully treat uveitic macular edema,” she said. Of note, she also recommended using OCT in the clinic to both detect and monitor macular edema in patients with uveitis.
—Patricia Weiser, PharmD
___________________________
1 Rathinam SR et al, for the FAST Research Group. JAMA. 2019;322(10):936-945.
2 Tsui E et al, for the FAST Research Group. Ophthalmology. Published online Feb. 8, 2022.
___________________________
Relevant financial disclosures: Dr Acharya—NEI: S.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Acharya NEI: S.
Dr. Ganguly None.
Dr. Lin None.
Dr. Patel AbbVie: C; Aerie: P; Emmecell: C; GlaxoSmithKline: C; Iris Medicine: P.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Hired to work for compensation or received a W2 from a company. |
| Employee, executive role |
EE |
Hired to work in an executive role for compensation or received a W2 from a company. |
| Owner of company |
EO |
Ownership or controlling interest in a company, other than stock. |
| Independent contractor |
I |
Contracted work, including contracted research. |
| Lecture fees/Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Patents/Royalty |
P |
Beneficiary of patents and/or royalties for intellectual property. |
| Equity/Stock/Stock options holder, private corporation |
PS |
Equity ownership, stock and/or stock options in privately owned firms, excluding mutual funds. |
| Grant support |
S |
Grant support or other financial support from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and\or pharmaceutical companies. Research funding should be disclosed by the principal or named investigator even if your institution receives the grant and manages the funds. |
| Equity/Stock/Stock options holder, public corporation |
US |
Equity ownership, stock and/or stock options in publicly traded firms, excluding mutual funds. |
More from this month’s News in Review
|