Download PDF
Clinics using websites to market “stem cell therapy” directly to the consumer—for everything from age-related macular degeneration (AMD) to retinitis pigmentosa (RP)—are proliferating, according to a study from the University of Rochester.1 And the consequences can be devastating.
“These sites are providing unapproved, untested therapies that have resulted in blinding complications,” said Ajay E. Kuriyan, MD, MS, at the University of Rochester’s Flaum Eye Institute in Rochester, New York. “With digital marketing, the ability to perform direct-to-patient marketing has evolved and allows more widespread dissemination” of bogus treatments.
There is a bit of good news, however: The FDA has won its suit against a Florida-based stem cell company that provided blinding treatments (see “Legal update”).
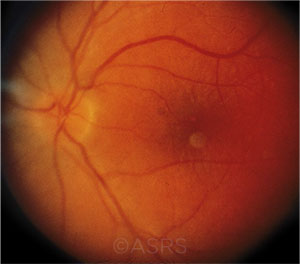 |
PATIENT DESPERATION. Optic neuritis (shown here), AMD, diabetic retinopathy, and retinitis pigmentosa are targeted by “cell therapy” clinics.This image was originally published in the ASRS Retina Image Bank. Maurice F. Rabb, MD. Optic Neuritis. Retina Image Bank. 2013; Image Number 11239. © The American Society of Retina Specialists.
|
Calling Dr. Google. For this study, Dr. Kuriyan and his colleagues performed an internet search using marketing terms such as “cell treatment” and “cell therapy.” During a two-week period in September 2017, they were able to identify 40 companies with 76 clinics in the United States that purported to treat ophthalmic conditions. Of the 40 companies, 35 offered treatment for AMD, followed by optic neuritis (n = 18), RP (n = 17), and diabetic retinopathy (n = 16).
The most frequently used cell type was autologous adipose-derived stem cells. Delivery methods included intravenous administration, injections, eyedrops, and “unspecified.” Most of the clinics did not disclose the cost of treatment; of the four that did, the cost per single treatment ranged from $4,000 to $10,500.
Risk of complications. Previously, Dr. Kuriyan and his colleagues reported on three patients who suffered blinding complications after receiving adipose-derived stem cells for AMD at a single clinic.2 Before the injection, the visual acuity of the patients’ better-seeing eyes ranged from 20/30 to 20/50. One year later, the VA of these eyes ranged from 20/200 to no light perception. Complications included retinal and vitreous hemorrhages, retinal detachments with proliferative vitreoretinopathy, and zonular weakness.
Talk to your patients. “Patients frequently ask about stem cell therapies for their condition,” Dr. Kuriyan said. “It is important to educate them and let them know about the differences between legitimate stem cell studies and these ‘stem cell’ clinics.”
Legal update. On June 3, a federal judge sided with the FDA in a lawsuit against U.S. Stem Cell Clinic, a Florida-based company whose treatments have blinded at least four patients. The judge affirmed that adipose-derived stromal vascular fraction cells can be considered a drug and thus are subject to FDA regulations.3
—Miriam Karmel
___________________________
1 Nirwan RS et al. Ophthalmology. Published online March 21, 2019.
2 Kuriyan AE et al. N Eng J Med. 2017;376(11):1047-1053.
3 www.fda.gov/news-events/press-announcements/federal-court-issues-decision-holding-us-stem-cell-clinics-and-owner-adulterated-and-misbranded-stem. Accessed June 18, 2019.
___________________________
Relevant financial disclosures—Dr. Kuriyan: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Holland None.
Dr. Kuriyan Alimera Sciences: C; Allergan: C; NEI: S; Regeneron: C; Roche/Genentech: S; Second Sight: S; Valeant: C.
Dr. Medeiros Allergan: C; Biogen: C; Carl Zeiss: C;S; Galimedix: C; Heidelberg Engineering: S; NEI: S; Ngoggle Diagnostics: P; Novartis: C; Reichert: C,S.
Dr. Peeler None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review