Download PDF
If the goal is to prevent endophthalmitis after phacoemulsification, an intracameral bolus of cefuroxime or moxifloxacin at the end of the surgery provides the most effective method, a large American study has concluded.
The publication of this study1 capped several years of efforts by Kaiser Permanente–based researchers to confirm what they found in a smaller preliminary study: that the predominant European method of prophylaxis also is appropriate for patients in the United States.2 The research also echoed the conclusions of a large Danish study published last year.3
What route; what drug? In the current study, the researchers analyzed the comparative effectiveness of various antibiotic regimens used by Kaiser Permanente ophthalmologists in 315,246 California surgeries over an 8-year period ending in 2012. The analysis showed that, compared with topical antibiotics, the adjusted odds ratio (OR) for incidence of endophthalmitis was 0.53 (95% CI, 0.30-0.95) in the intracameral cefuroxime eyes, and 0.68 (95% CI, 0.36-1.33) for those that received intracameral moxifloxacin.
“Surgeons who can change their approach to include intracameral injection of antibiotic should do so, because it better prevents endophthalmitis than does topical antibiotic,” said Lisa J. Herrinton, PhD, a coauthor of the National Eye Institute–funded study.
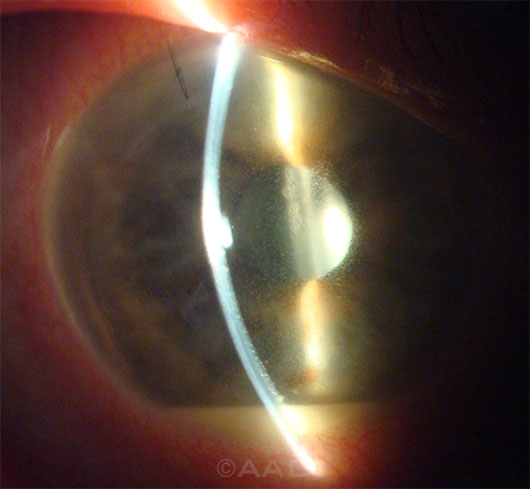 |
POSTOPERATIVE ENDOPHTHALMITIS. A large U.S. study corroborates earlier findings that endophthalmitis, like this case seen 4 days after cataract surgery, can be significantly reduced with intracameral antibiotics.
|
Findings on endophthalmitis risk. “We did not find a [significant] difference between intracameral moxifloxacin and intracameral cefuroxime. Either one is better than not using an intracameral agent,” said Dr. Herrinton, who is an epidemiologist at the Kaiser Permanente Division of Research, in Oakland, Calif.
Other key study results included the following:
- Posterior capsular rupture nearly quadrupled the infection risk, compared with uneventful phacoemulsification surgery (adjusted OR, 3.68).
- 4.5% of the study eyes received no antibiotic prophylaxis at all, as a result of either prescribing errors or patient noncompliance. The risk of endophthalmitis approximately doubled in these eyes, compared with eyes that received topical antibiotic therapy (adjusted OR, 1.95).
- The risk of endophthalmitis rose similarly in the 4.2% of eyes that were treated with a topical aminoglycoside, such as neomycin, gentamicin, or tobramycin (OR, 1.97). “Although aminoglycosides are effective against Staphylococci, the most common infective organism in post–cataract surgery endophthalmitis, evidence shows that they do not penetrate well into the anterior chamber,” the researchers explained.
- Three commonly used topical antibiotics (the fluoroquinolones gatifloxacin and ofloxacin, and polymyxin/trimethoprim) were equally ineffective at reducing endophthalmitis risk, compared with eyes that received no antibiotic therapy.
What about a combined approach? Would there be additional benefit from combining intracameral and topical prophylaxis? The study was not powered to answer that question, and most Kaiser Permanente surgeons who use intracameral antibiotics continue to prescribe topical medication after surgery, the researchers wrote.
Although that question remains unanswered, the authors stated: “Subconjunctival and topical antibiotics are intended to kill organisms on the ocular surface; however, aqueous concentrations might not be adequate to kill the most common causative organism, coagulase-negative Staphylococcus aureus. In contrast, intracameral antibiotics achieve concentrations that are several times greater than the concentration needed to kill 90% of most bacterial isolates.”
—Linda Roach
___________________________
1 Herrinton LJ et al. Ophthalmology. Published online Oct. 9, 2014.
2 Shorstein NH et al. J Cataract Refract Surg. 2013;39(1):8-14.
3 Kessel L et al. Acta Ophthalmol. 2015;93(4):303-317.
___________________________
Relevant financial disclosures—Dr. Herrinton: National Eye Institute: S.
For full disclosures and disclosure key, see below.
More from this month’s News in Review