Download PDF
Researchers at the University of Southern California (USC) hope the unique characteristics of their novel copolymer will solve an ophthalmic dilemma posed by combat- and mass-casualty–related ocular traumas. That is, scleral perforations sometimes must be left open for hours or even days before they can be repaired.
Thermoresponsive. The scientists chose a hydrophilic copolymer that was known to be thermoresponsive.1 This property makes the material suitable for temporarily closing open-globe wounds without further damaging the tissue, particularly when resources, facilities, or time are limited.
The researchers found that military ophthalmologists and other clinicians were able to rapidly and reversibly occlude scleral perforations in animal eyes with the compound, which is a polymeric combination of N-isopropylacrylamide and butylacrylate—also called poly(NIPAM-co-BA), or N95BA5.
“We [have tailored] its thermoresponsive behavior and mechanical strength to create a hydrogel that shape-fills upon injection at a wound site, adapting to irregular margins and sealing traumatic injuries. The thermosensitive behavior allows the sealant … to be easily removed by the application of cold water,” the authors wrote.
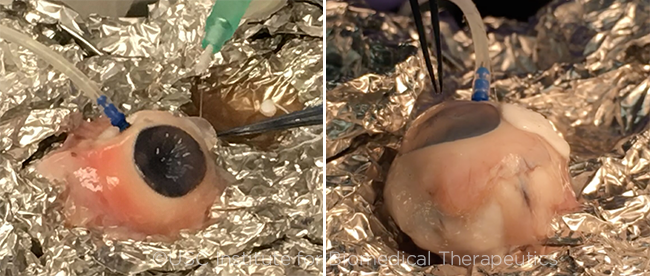 |
TEMPORARY CLOSURE. (Left) An ocular surgeon attempting to seal a large (~ 2 cm) full-thickness scleral laceration in a cannulated porcine eye using the ocular sealant and its associated customized injector tool designed by the team from USC. (Right) The same eye photographed 5 minutes later, with the sealant fixed in place and capable of maintaining IOP generated by the saline infusion cannula.
|
Material properties. Coauthor John J. Whalen, PhD, at USC’s Roski Eye Institute in Los Angeles, said that N95BA5 has the following properties:
- It exists as a viscous, translucent hydrophilic fluid at below 14 degrees Celsius (C). They designed a special double-walled (jacketed) syringe, capable of cooling the hydrogel on demand to below 10 degrees C for 10 minutes.
- It transitions to a more hydrophobic, opaque, sticky soft-solid state when body heat raises the temperature above 30 degrees C. This closes the wound and alleviates hypotony. (The solidification process takes approximately 5 minutes.)
- It returns to the liquid state when rehydrated with cold (< 10 degrees C) water, at which time the fluid can be aspirated away.
Early results. During in vivo testing in animals, the eyes showed some early signs of inflammation, which disappeared by 24 hours, the authors reported. There was no evidence of neurotoxicity, no retinal tissue degradation, and no significant chronic inflammatory response to sustained exposure (30 days).
Potential applications. Delayed treatment for open-globe injuries has added importance today because of traumatic eye injuries from explosions in war zones and in mass-casualty events, such as the Boston Marathon bombing. In the former scenario, scleral perforations are left open while patients are airlifted to a hospital, sometimes thousands of miles away. In the latter, care for more critically injured patients might take precedence over open-globe injuries, Dr. Whalen said.
Dr. Whalen, who is a bioengineering materials specialist on the USC research team that conducted research supporting the Argus II retinal implant (Second Sight), said the group originally investigated hydrogel polymers to reversibly adhere the Argus II to the retina. But serendipity pointed them to open-globe injuries instead.
“We couldn’t quite get this compound to work with the Argus. But when we saw that the Army was looking for temporary treatments for ocular trauma, we wondered if our adhesive could do the trick—and, from the first bench-top experiment, we had success,” Dr. Whalen said.
—Linda Roach
___________________________
1 Bayat N et al. Sci Transl Med. 2017;9(419). doi: 10.1126/scitranslmed.aan.3879.
___________________________
Relevant financial disclosures—Dr. Whalen: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Adrean Allergan: C,S; Genentech: C,S; Ohr Pharmaceuticals: C,S; Ophthotech: C,S; Regeneron: C,S; SciFluor Life Sciences: C,S.
Dr. Fieß None.
Dr. Medeiros Alcon: C; Allergan: C; Bausch + Lomb: S; Carl Zeiss: C,S; Heidelberg Engineering: C,S; Merck: S; NIH: S; Novartis: C; Sensimed: S; Topcon: S; Reichert: C,S.
Dr. Whalen None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review