By Linda Roach, Contributing Writer, interviewing Penny A. Asbell, MD, Charles W. Leiter, PharmD, David G. Miller, MD, and George A. Williams, MD
Download PDF
Pharmaceutical compounding has played an important role in ophthalmic practice for many years. However, a 3-year FDA crackdown on compounding pharmacies has narrowed ophthalmologists’ options for obtaining some of these medications.
The FDA’s regulatory framework now restricts the types and quantities of compounded drugs that small, local pharmacies can offer. It also restricts their volume of interstate shipments.1,2 Larger compounders with a nationwide customer base are being asked to register as “outsourcing” facilities subject to strict federal oversight.3
These changes are altering the pharmaceutical landscape and affecting options for patient care. Here’s a look behind the scenes at the pressures that pharmacies are under—and how this translates to increased expense (and scarcity) for physicians and patients.
 |
|
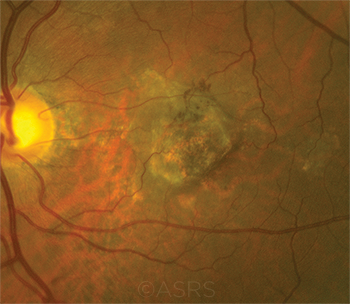
AMD COSTS. Current FDA regulations are adding to product development costs for pharmacies that compound bevacizumab (Avastin) for age-related macular degeneration (shown here). This image was originally published in the ASRS Retina Image Bank. Jason Griffith. Neovascular AMD. Retina Image Bank. 2017; Image Number 26899. © The American Society of Retina Specialists.
|
Drug Shortages
Pressure from these changes has made it increasingly difficult for physicians to acquire compounded products that were once easy to locate, said Penny A. Asbell, MD, MBA, at Mount Sinai Medical Center in New York, N.Y.
“It’s an ongoing struggle. There are just so many rules that it’s harder and harder to find a company that will do compounding,” Dr. Asbell said. “We used to frequently recommend autologous serum for patients with ocular surface disease. The patient would go to the pharmacy and have the blood drawn and made into eyedrops.” For instance, she said, her patients were once able to work with the pharmacy at Mount Sinai’s New York Eye and Ear Infirmary. “But now they no longer are authorized to make serum drops.”
David G. Miller, MD, a vitreoretinal specialist in Cleveland, said his practice had to stop using small, local compounders altogether because they no longer can send him medications without a patient-specific prescription. “People might be referred to me any day of the week, or even at night, with an infected eye after cataract surgery. I want to be able to treat that patient immediately with a compounded antibiotic that we keep on hand.”
Reports to the Academy. Members have reported problems acquiring compounded cefazolin, gentamicin, mitomycin, autologous serum eyedrops, tobramycin, and vancomycin for their practices, according to the Academy’s advocacy team in Washington.
Such shortages occurred after many state pharmacy boards, concerned about contaminants in compounded drugs, imposed the patient-specific prescription requirement on compounders in their states, said George A. Williams, MD, the Academy’s Secretary for Federal Affairs. “The effect is that it precludes ophthalmologists from having an office-based supply of drugs.”
Deaths Prompt Changes
Oversight of drug compounding tightened following the 2012 tragedy in which injections of a compounded steroid, contaminated by mold, caused fungal meningitis in more than 750 people, 64 of whom died. Trials for 2 officials of the now-defunct New England Compounding Center began early this year. In March, the jury in the first trial convicted former head pharmacist and co-owner Barry J. Cadden of fraud and racketeering (but not murder).
Two levels of pharmacies. This case highlighted weaknesses in the joint state-federal system of overseeing compounding pharmacies around the country, and it prompted Congress to pass the Drug Quality and Security Act in 2013. The effect was to establish a 2-tiered system of drug compounding, based on sections 503A and 503B of the FDA’s authorizing legislation.
503A. These are traditional, locally focused compounding pharmacies, which formulate customized drugs that are for specific patient needs and, in some states, only based on individual prescriptions. (This also allows compounding by physicians for their own patients.) Under the guidelines, oversight of these pharmacies would remain primarily a state function, unless the FDA received a complaint.
503B. These are outsourcing pharmacies, which can register with the FDA and make large batches of compounded drugs, test them for sterility and quality, and—without individual prescriptions—supply compounded drugs to physicians and medical facilities. Registered 503B pharmacies must meet current good manufacturing practices and are subject to regular federal inspections, just as conventional drug manufacturing plants are.
Continuing Uncertainty
Details about how the FDA will implement the new system have emerged slowly over the last 2 years in a series of “draft guidances,” many of which have not been finalized. The drawn-out process has caused uncertainty and an abundance of caution among compounding pharmacists and the physicians who rely on them, said Charles W. Leiter, PharmD, whose Leiter’s pharmacy in San Jose, Calif., has provided compounded preparations to ophthalmologists nationwide for many years.
Limits on “just-in-case” Rx. State rules for 503A compounding pharmacies are still preventing some small, local compounders (including hospital pharmacies) from providing ophthalmologists with supplies of fortified antibiotics and other commonly compounded drugs for urgent cases, Dr. Williams said. However, the Academy has made progress addressing this issue, he reported.
“The pharmacy boards are acting in good faith. They think that it’s a patient protection issue,” Dr. Williams said. “Most states have rolled this back. There are just a few states where that is still active, and the Academy is assessing with their pharmacy boards the issues that this creates. We have to convince them that it’s probably not engendering as much safety as they believe and that it’s actually causing some harm in selected patients.”
For registered 503B compounders, financial barriers are being imposed at the federal level, Dr. Leiter said. The proposed guidances permit them to send doctors anticipatory shipments of these compounded medicines, but only after the pharmacy performs expensive tests to verify their expiration dates.
Harder to ship across state lines. In the wake of the multistate fungal meningitis outbreak, regulators in many states began requiring out-of-state pharmacies to obtain licenses to ship their compounded products there, Dr. Asbell said. In her practice, the state rules have made lissamine green and fluorescein for evaluating dry eye hard to obtain, she said. “We’re now on about the fourth pharmacy.”
The problem for lissamine green, Dr. Leiter noted, is that the pharmacies are no longer able to compound the product because it doesn’t meet 3 requirements in the FDA rules: It is not available to compounders in USP-grade chemical form, there is no FDA monograph covering it, and it is not on the FDA’s “OK to compound” list.
Impact on networks. When a health system pharmacy sends compounded medications without patient-specific prescriptions to other sites in the system, the receiving facilities should be no more than 1 mile away, the FDA says.4 This is because a pharmacy that distributes compounded drugs across a wider area “could function as a large manufacturing operation, but without the necessary standards to assure drug quality,” the draft guidances say.
Shorter compendia. Pharmacies that have registered for 503B status (58 as of January5) can formulate and ship drugs without individual prescriptions. But ophthalmologists may find that their preferred sterile compounder has slashed the number of drugs it makes—or has plans to trim the list when the FDA rules for 503B compounders become finalized. The reasons are largely economic, said Dr. Leiter.
$50,000 per drug. The FDA guidelines state that each 503B-compounded medication should undergo extensive baseline testing (inside the intended container) to prove the packaged product’s sterility and stability before the drug can be marketed—and these tests are costly, Dr. Leiter said. Indeed, the complexity and costs of establishing and maintaining compliance with the new FDA guidances convinced Dr. Leiter and his family to sell a majority interest in the firm 2 years ago to a private equity group, he said.
“In a 503B facility, if I’m going to make something, I have to spend $50,000 [to test it],” Dr. Leiter said. “I used to have an 1,800-drug compendium. If I spent $50,000 on each one of them, I’d be spending $90 million on testing.” Now the company’s website has just 11 ophthalmic compounds on its product list. “We’d like to get up to about 30 products,” he said.
What About Avastin?
In 2015, both the Academy and the American Society of Retina Specialists encouraged members to comment when the FDA’s draft document on biologics proposed a “beyond-use date” of 5 days for compounded biologics. This would have effectively ended the availability of bevacizumab (Avastin) in ophthalmology, Dr. Leiter said.
Victory for ophthalmologists? The agency’s revised guidances for biologics, released in January, are open ended.3 They would allow 503B pharmacies to determine the expiration dates on their bevacizumab syringes by conducting stability tests. This was a win for ophthalmologists and their patients, but it will necessitate another expensive testing regimen for compounders, Dr. Leiter said. “We’ll have to perform about $100,000 worth of testing in order to get a longer shelf life on the label.”
Squeeze on practices. Dr. Miller said he was pleased that the FDA dropped its 5-day rule, but he is concerned that the added testing expense might have an impact on his vitreoretinal practice.
Dr. Williams agreed. “Already we’re hearing from around the country that certain suppliers have priced Avastin at well above the Medicare reimbursement rate. At some point, people have to ask themselves what financial risks they are willing to take.”
Ultimately, the impact of the regulations will fall hardest on patients, Dr. Leiter said. “What about the people who have rare diseases and can’t get their medicine? Even if 50 people a year get that disease, you’re still going to have to do tens of thousands of dollars of testing before you could provide it to them. And with these new laws, I couldn’t even afford to give it to them for free.”
___________________________
Note: Guidance documents in citations 1-4 are available at: https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/PharmacyCompounding/ucm166743.htm.
___________________________
1 Food and Drug Administration. Pharmacy Compounding of Human Drug Products Under Section 503A of the Federal Food, Drug, and Cosmetic Act: Guidance. Issued Oct. 26, 2015.
2 Center for Drug Evaluation and Research. Prescription Requirement Under Section 503A of the Federal Food, Drug, and Cosmetic Act: Guidance for Industry. Issued Dec. 28, 2016.
3 Food and Drug Administration. Mixing, Diluting, and Repackaging Biological Products Outside the Scope of an Approved Biologics License Application: Revised Draft Guidance. Issued Jan. 12, 2017.
4 Food and Drug Administration. Hospital and Health System Compounding Under the Federal Food, Drug, and Cosmetic Act: Guidance for Industry. Issued April 15, 2016.
5 Food and Drug Administration. Registered Outsourcing Facilities. Updated Feb. 17, 2017.
___________________________
Dr. Asbell is a professor of ophthalmology and director of the Cornea Service and Refractive Surgery Center at Mount Sinai Medical Center in New York. Relevant financial disclosures: None.
Dr. Leiter is vice president for business development at Leiter’s in San Jose, Calif. Relevant financial disclosures: Leiter’s: O.
Dr. Miller is a vitreoretinal specialist with Retina Associates of Cleveland. Relevant financial disclosures: Regeneron: C.
Dr. Williams is a vitreoretinal specialist in southeastern Michigan and the Academy’s Secretary for Federal Affairs. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.