Download PDF
Where’s the epinephrine? The shortage of preservative-free, bisulfite-free epinephrine—used in cataract surgery for its ability to maintain mydriasis—began last year and shows no signs of ending any time soon. Some cataract surgeons have coped by using epinephrine that contains bisulfites, but even that form has been difficult to come by recently.
Although the U.S. supplier of preservative- and bisulfite-free epinephrine (American Regent) says that manufacturing has resumed and restocking is expected, at the time of publication, it had not guaranteed a resupply date (see “Keep Up to Date”). As a result, cataract surgeons around the country continue to investigate alternatives.
|
Tamsulosin Complications
|
 |
|
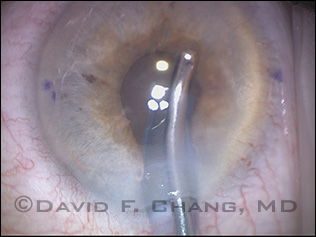
Miosis and iris prolapse into the paracentesis and main incision during I&A in a tamsulosin patient with IFIS.
|
Epinephrine: Most Popular, Mostly Unavailable
When it is available, preservative-free epinephrine comes in two forms: with and without 0.1 percent bisulfite. “Bisulfite 0.1 percent is commonly used to stabilize commercially available ampules of 1:1,000 epinephrine,” said David F. Chang, MD, in practice in Los Altos, Calif., and at the University of California, San Francisco. However, “When this undiluted drug is injected directly into the anterior chamber, the bisulfite is toxic to corneal endothelial cells because of its high buffer capacity. This led to the theoretical preference for using bisulfite-free 1:1,000 epinephrine for any direct intracameral injection.”
In recent years, this theoretical preference became a practical one, as researchers reported that preservative- and bisulfite-free epinephrine was effective in maintaining mydriasis during surgery on patients with intraoperative floppy iris syndrome (IFIS).1 “Most of us use the mixture of lidocaine and epinephrine known as epi-Shugarcaine,” said Sonia H. Yoo, MD, at Bascom Palmer Eye Institute. “It is particularly helpful for IFIS.”
But that changed early last year, when American Regent stopped manufacturing preservative- and bisulfite-free epinephrine, and cataract surgeons had to scramble for alternatives. “Many concerns have arisen because it has been unavailable during the past year,” Dr. Chang noted.
Surgical Tips
Intracameral epinephrine and phenylephrine are popular and effective adjuncts for managing potential IFIS, the experts note. They offered the following tips for their use.
Before surgery. Tamsulosin (Flomax) is the best-known drug for treating benign prostatic hyperplasia (BPH), but another one to be aware of is silodosin (Rapaflow). Like Flomax, Rapaflow is a selective antagonist for the alpha1A subtype and is associated with a greater incidence of IFIS than are the other BPH drugs.
During surgery. Although intracameral epinephrine and phenylephrine “may not necessarily increase mydriasis, they may still prevent or mitigate the degree of intraoperative miosis,” Dr. Chang said. “In addition, the alpha-agonists increase the rigidity of the iris stroma, which lessens the tendency for iris prolapse.”
Many surgeons use a stepwise approach for mydriasis in patients taking systemic alpha-antagonists such as Flomax, Dr. Chang said. “If the pupil remains too small following alpha-agonist injection, or if additional surgical risk factors are present, mechanical dilation with iris retractors or a pupil expansion ring, such as the Malyugin ring, will assure a sufficient pupil diameter for phaco,” he said.
Dr. Henderson agreed. “I find that intracameral epinephrine is helpful in dilating the pupil in most cases. However, in some eyes, it is not enough. In cases where the pupil does not dilate sufficiently, I will use either iris hooks or a ring.”
|
What Now? The Alternatives
Epinephrine with bisulfites. “Once we knew we no longer had a supply of preservative-free, bisulfite-free epinephrine, then the question naturally arose: Is it okay to use the version that contains bisulfite?” Dr. Yoo said. Based on increasing anecdotal experience, the answer appears to be yes—as long as it is diluted appropriately.
“Bisulfite-containing 1:1,000 epinephrine can be safely injected intracamerally if it is diluted 1:4” with either balanced salt solution (BSS) or fortified BSS (BSS Plus), Dr. Chang said. Anecdotally, Dr. Yoo said, a number of cataract surgeons have been using it that way without inciting toxic anterior segment syndrome.
Initially, this was also Dr. Chang’s preferred solution to the problem. “With the shortage of bisulfite-free epinephrine, I have used bisulfite-containing epinephrine diluted 1:4 with BSS in many eyes without any sign of endothelial toxicity.”
However, earlier this year, he ran into a second epinephrine shortage. “For a time, bisulfite-containing epinephrine also became unavailable, leaving us without epinephrine to add to the BSS irrigation bottle.” When this happened, Dr. Chang, like other cataract surgeons, immediately noticed an increase in the incidence of IFIS, even in patients who were not taking one of the alpha-antagonist drugs that have been linked to the condition.2
Phenylephrine. Faced with an expanding epinephrine shortage, some cataract surgeons began turning to phenylephrine. “I don’t use phenylephrine, but many surgeons do,” said Bonnie A. Henderson, MD, in practice in Boston.
Some studies have suggested that the use of preservative-free, bisulfite-free phenylephrine, administered with or without lidocaine, provides adequate pupil dilation and is effective in preventing IFIS.3
For instance, in a study published last year, researchers in Spain compared patients receiving the intracameral combination of phenylephrine and lidocaine with those receiving only BSS during phacoemulsification. All of the 42 patients in the study were taking tamsulosin (Flomax), the drug most often implicated in IFIS. The incidence of IFIS was zero in those patients receiving phenylephrine, versus 88 percent in those receiving BSS alone.4
However, preservative-free, bisulfite-free phenylephrine is not commercially available in the United States. In Europe and other parts of the world, cataract surgeons have the option of using commercially available bisulfite-free phenylephrine and diluting it 1:4 with BSS, BSS Plus, or preservative-free lidocaine (as in the Spanish study cited above). In the United States, however, ophthalmologists must use a compounding pharmacy to obtain a preservative- and bisulfite-free formulation of the drug.
Dr. Chang now uses 1.5 percent phenylephrine mixed with 1 percent lidocaine, compounded by Leiter’s Pharmacy in San Jose, Calif. “When we use epinephrine, we directly dilute it with BSS in a 3-mL syringe. The compounded 1.5 percent phenylephrine requires no further mixing.” He added, “The compounded solution is inexpensive, has a shelf life of more than two months, and is very effective for IFIS,” he said. A similar mixture is being used at Bascom Palmer, Dr. Yoo reported.
Keep Up to Date
For updates and safety information, consult the following resources.
Drug shortages. American Regent is posting news on its production schedule at www.americanregent.com. The American Society of Health-System Pharmacists has useful updates on drug shortages at www.ashp.org.
Compounding pharmacies. The PCAB lists accredited compounding pharmacies by state and name at www.pcab.org. (These pharmacies are regulated by state pharmacy boards, even though they sell across state lines.) The FDA has information on compounding pharmacies at www.fda.gov and updates at its “FDA Voice” blog.
|
Safety Concerns
However, given recent industry issues, many surgeons may be reluctant to consider a compounding pharmacy. Dr. Chang understands that point of view. “Recent drug recalls from compounding pharmacies because of microbial contamination have shaken the confidence of many patients and physicians in compounded medications.” In ophthalmology, for instance, contaminated versions of trypan blue, brilliant blue G, and bevacizumab (Avastin) have been linked to endophthalmitis and blindness.5
Despite attention to the problem, it hasn’t yet been completely resolved: In mid-April, the FDA conducted a “crash inspection” of compounding pharmacies and reported finding a number of unsafe and unsterile conditions.6
“You need to have some quality control regarding sterility,” Dr. Yoo cautioned. At Bascom Palmer, she said, “We sterilize our mixture through a micropore [filter] and then test 10 percent of it through the microbiology department. The microbiology laboratory will plate the mixture and then hold the plates for 14 days, monitoring them daily. That’s how we ensure that our stock is sterile. If you aren’t in an academic setting and don’t have the luxury of your own lab, you have to have assurance that it’s sterile.”
But given current drug shortages, working with a compounding pharmacy is “important and necessary,” Dr. Henderson said, and she noted that individual compounding pharmacies differ from one another. Drs. Chang and Henderson also recommended that any ophthalmologist who is pursuing this option should check to see whether the compounding pharmacy is accredited by the Pharmacy Compounding Accreditation Board, or PCAB (see “Keep Up to Date”). “One can also request a ‘certificate of sterility’ from a compounding pharmacy for a new intracameral preparation,” Dr. Chang said.
Finally, when it comes to ordering phenylephrine from a compounding pharmacy, ophthalmologists should be careful to specify that only the unpreserved (raw) drug should be used.
___________________________
1 Chang DF et al. J Cataract Refract Surg. 2008;34(12):2153-2162.
2 Clinical Alert. Alpha-agonist formulations for intracameral use. American Society of Cataract and Refractive Surgeons, March 2013. Accessed April 12, 2013.
3 Clinical Statement. Drug shortage: nonpreserved, bisulfate-free epinephrine. American Academy of Ophthalmology, December 2012. Accessed April 12, 2013.
4 Lorente R et al. Ophthalmology. 2012;119(10):2053-2058.
5 Resources: Compounded drugs and off-label use. Ophthalmic Mutual Insurance Company (OMIC). Accessed April 12, 2013.
6 Proactive inspections further highlight need for new authorities for pharmacy compounding. U.S. Food and Drug Administration. April 11, 2013. Accessed April 12, 2013.
___________________________
David F. Chang, MD, is in private practice in Los Altos, Calif., and is a clinical professor of ophthalmology at the University of California, San Francisco. Financial disclosure: Is a consultant for Abbott Medical Optics, Clarity, LensAR, and Transcend; receives lecture fees from Allergan and Glaukos; owns equity in Calhoun Vision, Clarity, ICON, LensAR, PowerVision, Revital Vision, Transcend, and Versant Ventures; and has patent/royalty interest in Eyemaginations and Slack. Bonnie A. Henderson, MD, is in practice with Ophthalmic Consultants of Boston. Financial disclosure: Is a consultant for Alcon and Bausch + Lomb and has royalty interest in the Virtual Mentor cataract training system. Sonia H. Yoo, is a professor of ophthalmology at Bascom Palmer Eye Institute, Miami. Financial disclosure: Is a consultant for Alcon, Bausch + Lomb, Optimedica, and Transcend; receives lecture fees from Alcon and Slack; and receives grant support from Allergan and Carl Zeiss Meditec.