Download PDF
Now that corneal cross-linking has received FDA approval, will clinical practice outpace evidence-based protocols?
For almost 20 years, researchers have proposed corneal cross-linking (CXL) as a method to stabilize the cornea in patients with diseases that involve progressive corneal thinning, notably keratoconus (KC). Although the procedure has been available abroad since 2007, the FDA held back on approval in the United States because the current body of research lacks standardization.1
That changed last year when the FDA cleared Avedro’s KXL system and 2 photoenhancers, Photrexa and Photrexa Viscous, despite the nonstandardized data, citing the “unmet need” for the KC treatment.1
Game Changer
The FDA’s step is “a game changer,” said Bennie H. Jeng, MD, at the University of Maryland School of Medicine in Baltimore. CXL “is the first available modality that can halt the progression of keratoconus. If we’re able to reach patients at a stage where they have decent vision and freeze them in that position for the rest of their lives, that’s groundbreaking.”
Indeed, the promise of CXL is the first generation of patients who will not routinely need keratoplasty or suffer progressive vision loss. Two European studies have already reported significant reductions in the number of corneal transplants for KC since the introduction of CXL there.2,3 Though the studies are retrospective, they are still encouraging, Dr. Jeng said. “The one variable that has not been factored in is improved contact lens technology, which has also decreased the rate of keratoplasty for KC,” he said.
“I believe CXL is one of the most important innovations in anterior segment surgery since the introduction of excimer lasers,” said Alaa M. ElDanasoury, MD, at Magrabi Eye and Ear Hospitals in Saudi Arabia. “Our American colleagues have an excellent opportunity to learn from international experience and decrease their learning curve,” added Dr. ElDanasoury, who has performed the procedure since 2005 on more than 2,000 eyes.
An Overview of CXL
CXL is a photochemical reaction of corneal collagen resulting from the combined action of the photosensitizer riboflavin with ultraviolet A (UVA) irradiation. It is a minimally invasive procedure that stiffens the anterior corneal stroma by creating strong covalent bonds between collagen fibrils.
What’s approved. The Avedro KXL system received FDA approval in April 2016 for the indication of progressive KC. In July 2016, it was approved for the treatment of corneal ectasia after refractive surgery.
Using the Avedro system for other indications is considered an off-label application. In addition, the use of CXL systems from other device companies is considered experimental by the FDA, requiring institutional review board (IRB) and Investigational New Drug (IND) approvals. (See “Malpractice Policies: 4 Key Questions,” below.)
The protocol. The standard CXL protocol, known as the Dresden protocol, involves initial removal of the central corneal epithelium to allow better diffusion of the riboflavin into the stroma.
After de-epithelialization, a 0.1% riboflavin solution is applied to the cornea—every 1 to 3 minutes for 30 minutes, in a process called imbibition—until the stroma is completely penetrated. The cornea is then irradiated for 30 minutes with 370 nm UVA (a maximal wavelength for absorption by riboflavin) at a power of 3 milliwatts (mW)/cm2.4
This protocol is effective at cross-linking the anterior 300 μm of the cornea and halting the progression of KC in most cases, Dr. ElDanasoury said. “The failure rate [progression of KC] is less than 2%.”
Refractive Correction for KC
KC is a bilateral, progressive corneal steepening and stromal thinning that impairs visual acuity and typically starts during the second decade of life. Progression is highly variable until the fourth decade, when corneal shape usually stabilizes.5,6
Lenses. For patients with mild KC, glasses may be enough for vision correction. Contact lenses, usually soft toric lenses to correct astigmatism, may be needed.
But many patients feel that glasses or soft lenses are insufficient, so Christopher J. Rapuano, MD, then tries rigid gas-permeable contact lenses. “Most patients are satisfied with their vision in rigid gas-permeable contact lenses, but there are other lenses that can be tried,” said Dr. Rapuano, at Wills Eye Hospital and Jefferson Medical College in Philadelphia. These include hybrid lenses, which are hard in the middle to give good vision with a soft skirt on the outside.
A piggyback system is also an option. In this scenario, a soft lens goes on the eye and then a hard lens is placed on top of that to make it more comfortable for the patient. Finally, scleral lenses, which are big lenses that vault over the cornea, can be tried. “These lens options often provide very good vision and help patients avoid surgery,” said Dr. Rapuano.
Intracorneal rings. When a patient doesn’t do well with contact lenses and there’s not too much scarring or thinning, Dr. Rapuano considers implanting intrastromal corneal ring segments (ICRS) in the peripheral cornea to reshape the cornea. “Typically, we try ICRS in patients who were doing well in contacts but got a little worse and now can’t wear the contacts anymore. We’re just trying to help them move back into their contacts.”
Corneal transplants. If ICRS don’t work or if there’s too much scarring in the cornea, the final step is a corneal transplant. Some patients can do well with a top-layer transplant (deep anterior lamellar keratoplasty, or DALK). Alternatively, a full-thickness transplant (penetrating keratoplasty, or PK) may be performed. DALK is preferred because it decreases rejection risk. “The downside is that it’s technically more difficult,” said Dr. Rapuano, “and the Descemet membrane often tears during surgery, so you go on to do a PK anyway.”
When to consider CXL. The modalities listed above attempt to improve refractive errors but are not disease modifying. “We are always concerned about progression of KC, especially in younger patients and those who don’t yet have severe KC. We follow them pretty closely—and if we think the disease is getting worse, then we consider CXL,” said Dr. Rapuano. “In practical terms, we have had nothing that stops or slows the progression of KC other than telling patients to stop rubbing their eyes. CXL ushers in a new era of KC management.”
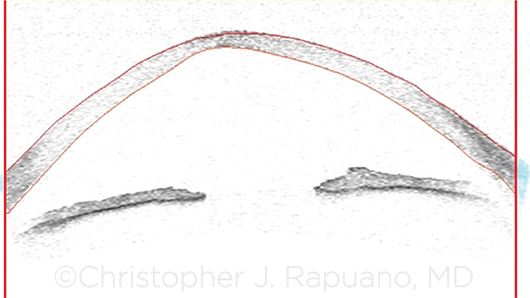 |
|
OCT. Anterior segment optical coherence tomography image of an eye with KC demonstrates central corneal thinning, posterior bowing, and anterior steepening.
|
Preferred Candidates for CXL
“Just because a patient has KC doesn’t mean he or she needs CXL,” said Dr. Jeng. “Patients who should be referred for evaluation for CXL are those who have demonstrated progression, especially a teenager or someone who stands to gain the most from the procedure,” he said.
However, as Dr. Jeng cautioned, “The tricky part is determining who is at risk for progression. If KC is stable, such as in a 50-year-old patient, you don’t need CXL.” The criteria for progression of ectasia are not well defined, but changes in refraction, uncorrected visual acuity [VA], best-corrected VA, and topographic parameters are typically used.
Patients with KC. A good candidate is between the ages of 15 and 35 and has a minimum corneal thickness of approximately 440 to 450 μm before the procedure with the epithelium on, Dr. Rapuano said.
Patients with postrefractive ectasia. For CXL among patients experiencing progressive curvature changes after refractive surgery (typically post-LASIK),5 age is not a factor. “Results around the world are pretty good for postrefractive ectasia, though not as strong as seen with regular KC,” Dr. Rapuano said.
Contraindications. Standard CXL treatment is not an option when corneal thickness is less than 400 μm; when severe corneal scarring or opacification is present; when there is a history of herpetic infection, poor epithelial wound healing, or immune disorders; or when a patient has severe ocular surface disease, is pregnant, or is breastfeeding.5
What about children with KC? Despite the off-label status of CXL in children under age 14, the experts interviewed for this article said they would perform the procedure in a child as young as 10 if there is documented progression of KC. Data on CXL in the pediatric population are limited but thus far show comparable results to adults, with a good safety-efficacy profile during follow-up periods of up to 3 years.7
The timing of CXL in children is controversial. Some surgeons advocate doing the procedure right away. Our interviewees recommend monitoring children very closely to watch for the earliest signs of progression. At that point, CXL can be offered.8
Dr. ElDanasoury’s youngest patient to date was 11 years old at the time of CXL, and he followed the standard Dresden protocol. “Now, at age 17 with stable KC, the patient is enjoying good, functional corrected distance VA,” Dr. ElDanasoury said.
Getting started. J. Bradley Randleman, MD, at the University of Southern California’s Roski Eye Institute in Los Angeles, offers CXL to patients with KC or post-LASIK ectasia. He is in a unique position, having been involved in CXL since the original U.S. clinical trial in 2008. “For ophthalmologists just starting with CXL, I recommend beginning with patients you can identify as having progressive KC with reasonable corneal thickness and with reasonable expectations about the treatment,” said Dr. Randleman. “CXL is highly effective at halting the progression of KC, but it is not a cure and will have limited impact on the patient’s refraction when performed in isolation.” (See “Handling Patient Expectations,” below.)
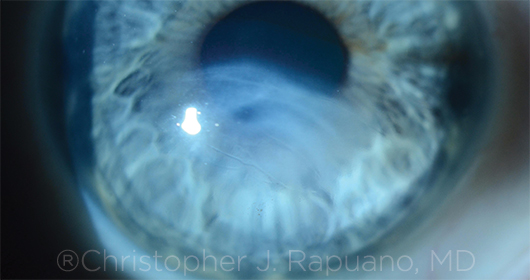 |
|
PROGRESSION. Significant scarring after hydrops in an eye with progressive KC.
|
Evolving Techniques
Although the Dresden protocol is supported by the most research and clinical experience, it has 2 major disadvantages, especially in children, for whom cooperation and compliance are major issues: 1) the duration of the procedure, which runs approximately 1 hour, and 2) the removal of the epithelium to allow the riboflavin to penetrate the deeper corneal layers. De-epithelialization results in postoperative pain and a higher risk of infection, melting, scarring, and haziness, Dr. Rapuano said.
To address these disadvantages, investigators have explored the following variations.
Accelerated CXL. One option is to reduce exposure time by increasing the power density of the irradiation.5 There is some evidence that the anterior 300 μm of the cornea can be cross-linked to stabilize KC by increasing the power density to 9 mW/cm2 and decreasing irradiation time to 10 minutes, Dr. ElDanasoury said. Some surgeons have used “flash cross-linking,” which involves the delivery of even higher power UVA for a shorter duration, but the CXL effect was limited to superficial layers of the cornea, he noted.
Transepithelial CXL. Investigators also have searched for methods to keep the epithelium on, by using different compositions of riboflavin solution to try to penetrate the cornea with an intact epithelium. Studies have shown that transepithelial (epi-on) CXL has only a superficial effect, although research is being conducted on strategies to improve penetration.5
Follow the evidence. For now, the interviewees advise, stick with the Dresden protocol. Given the relative paucity of clinical evidence, the most effective method to halt the progression of KC is to follow evidence-based protocols. “Learn to walk before running with the procedure,” Dr. Randleman urged.
“It is not worth trying to save a few minutes or decrease postoperative pain at the expense of treatment efficacy,” said Dr. ElDanasoury. He advised that CXL for progressive KC be performed with the epithelium off for 30 minutes with a power of 3 mW/cm2 or at least 10 minutes with a power of 9 mW/cm2.
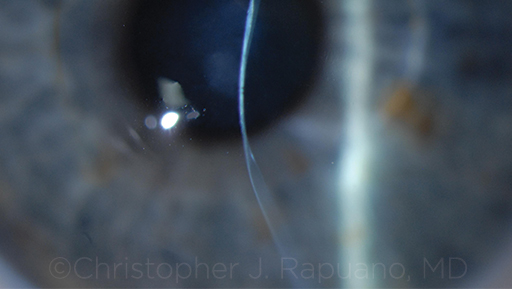 |
|
CONTRAINDICATED. Severe corneal thinning and moderate scarring in an eye with advanced KC. This eye would not be a candidate for CXL.
|
Off-Label Use
Off-label applications—in addition to the use of CXL in children under the age of 14—currently include treatment of infectious keratitis and combination surgery; the latter is known as “CXL plus.”
Infectious keratitis. CXL kills a range of microbes by causing irreversible damage to their RNA and DNA. The idea behind using CXL to treat infection is to strengthen the cornea while killing the infectious organisms.5 Studies have reported positive results for patients with some multidrug-resistant bacteria and for some patients with fungal keratitis, but results have been mixed for Acanthamoeba, Dr. Rapuano said. The depth of the ulcer is a major factor. “CXL certainly seems to help superficial and moderately deep infections but not very deep ones,” he said.
Dr. ElDanasoury uses CXL for cases of infectious bacterial keratitis when patients have a poor response to antibiotics. “For cases of fungal keratitis, we perform CXL even earlier with relatively good results.” (For more about CXL to treat infection, see “Potential of Cross-Linking for Corneal Infection.”)
CXL plus. While the main objective of CXL is to “stiffen” the cornea, secondary effects such as topographic and refractive changes also may occur. In some cases, these changes may be advantageous, as they help improve corneal shape, decreasing the myopia typically associated with KC, as Dr. ElDanasoury has found. However, the changes are unpredictable, failing to correlate with any preoperative parameters. Nonetheless, surgeons have started combining the procedure with other interventions.
CXL plus ICRS. CXL can halt KC progression but doesn’t typically improve vision. In contrast, ICRS can produce significant improvements in vision but do not stop KC progression. Although, theoretically, combining the procedures could produce better results, studies have reported mixed results, and the best approach is still undefined.5
CXL plus LASIK or PRK. KC has long been considered a contraindication for LASIK or PRK, but the possibility of doing the surgery in patients with stable KC has been proposed in recent years. The optimal timing and interval between the procedures needs further investigation, given that the refractive effect of CXL is unpredictable and poorly understood.5 “It is tenuous at best to believe that adding CXL to LASIK or PRK has no short- or long-term effects on the refractive outcome of excimer surgery, which requires almost submicron precision,” said Dr. ElDanasoury.
Some surgeons have performed CXL as a prophylactic measure against postrefractive ectasia by treating LASIK patients with high-power UVA for a very short period (1 minute) after a 1-minute riboflavin imbibition time. This procedure has been termed LASIK Xtra. “There is no clinical evidence that LASIK Xtra prevents ectasia, but it increases the risk and cost of the procedure,” said Dr. ElDanasoury.
Handling Patient Expectations
It is essential for patients to understand that CXL has limits.
Not a miracle. “The treatment is not intended to make your vision better; it is intended to stop the progression of KC to enable you to retain the vision you currently have with the [refractive] modality you are using to see,” Dr. Jeng said. “If you’re in glasses and progressing, it should be able to keep you in glasses. If you’re in [contact] lenses, it should be able to keep you in lenses without having to go to the next step, which is surgery.”
It’s also important to tell the patient that there is no guarantee that CXL will work. “It works in most people but not everyone,” said Dr. Jeng.
Treatment timing. Dr. Rapuano added that patients should be aware that 1 eye is done at a time. They should be prepared to be out of commission for the first week after surgery and be aware that it’s painful for that first week.
Consent issues. If a clinician plans to use the Avedro system for off-label applications, Anne M. Menke, RN, PhD, advised discussing the off-label status of the procedure with the patient. Similarly, the clinician should notify the patient if another device and/or riboflavin solution will be used. “Document these discussions appropriately in the medical record and informed consent document,” said Dr. Menke, a risk manager at the Ophthalmic Mutual Insurance Company (OMIC) in San Francisco.
CXL Tomorrow
In practical terms, Dr. Jeng hopes that with FDA approval, patients’ health insurance plans will start to cover the procedure, making it more accessible. Dr. ElDanasoury expects some major developments in upcoming years, including the following:
- Ongoing research on the ablation rate of the cross-linked cornea that may allow safe and predictable PRK after stabilization of the KC and refractive error post-CXL.
- New riboflavin solutions that can effectively cross the epithelial barrier and achieve deeper corneal penetration with the epi-on technique, to make the procedure less painful.
- Greater understanding of the biomechanical effect of CXL, which may make the refractive change more predictable.
- Customized CXL, which is currently under investigation. This procedure, once adequately developed, will treat specific areas on the cornea that require cross-linking, sparing normal corneal tissue.
___________________________
1 Jeng BH et al. Ophthalmology. 2016;123(11):2270-2272.
2 Sandvik GF et al. Cornea. 2015;34(9):991-995.
3 Godefrooij DA et al. Acta Ophthalmol. 2016;94(7):675-678.
4 Wellensak G et al. Am J Ophthalmol. 2003:135(5):620-627.
5 Mastropasqua L. Eye Vis (Lond). 2015;2:19. doi:10.1186/s40662-015-0030-6.
6 Gomes JA et al.; Group of Panelists for the Global Delphi Panel of Keratoconus and Ectatic Diseases. Cornea. 2015;34(12):359-369.
7 El Rami H et al. Biomed Res Int. 2015;2015:257927. doi:10.1155/2015/257927.
8 Kankariya VP et al. Indian J Ophthalmol. 2013;61(8):435-440.
Malpractice Policies: 4 Key Questions
The advent of CXL in the United States has raised myriad concerns regarding the potential for malpractice claims. Here are 4 key questions—and their answers—supplied by OMIC’s Dr. Menke. (As insurance policies differ, be sure to check with your provider if you are not covered by OMIC.)
1. Are off-label uses of the Avedro KXL system covered by my malpractice policy?
Short answer: Yes.
Discussion: OMIC considers off-label use in the treatment of an individual patient to be a legal and necessary part of the practice of medicine. The FDA has indicated that physicians may use approved drugs and devices off label for other purposes as part of the practice of medicine if they 1) are well informed about the product, 2) base its use on firm scientific method and sound medical evidence, and 3) maintain records of its use and effects.1
The distinction between “off-label” and “unapproved” is not always clear. The use of some unapproved devices may be common practice and considered low risk, while some off-label uses might be considered risky for the patient or deemed to constitute research. We encourage physicians to conduct a risk assessment and to check with their insurance carrier.2
2. Is the use of a non-FDA–approved (i.e., non-Avedro) cross-linking machine covered by my malpractice policy? What about non-FDA–approved riboflavin solutions?
Short answer: Yes, with an IND and IRB approval.
Discussion: OMIC policyholders who use any device other than the Avedro KXL System or riboflavin solutions other than Photrexa and Photrexa Viscous must do so under and in accordance with a U.S. IRB-approved protocol and obtain an IND approval from the FDA for the riboflavin solution until such other device or riboflavin solution obtains FDA approval for CXL. A single IND approval may be used for multiple solutions and/or devices. IRB approval may be obtained before, during, or after the IND approval becomes effective.
3. Does technique impact the ophthalmologist’s malpractice coverage?
Short answer: No.
Discussion: Coverage for CXL is not dependent upon the technique. We know that clinical questions remain. The optimal technique to use in CXL is one of the key questions being analyzed in ongoing clinical trials and research.
4. What advice do you have about consent forms?
Short answer: Use them!
Discussion: While lack of informed consent is rarely the main or only allegation in a medical malpractice lawsuit, it surfaces regularly. Using a procedure-specific consent form reduces the likelihood and success of such allegations. Just as important, procedure-specific consent forms help educate the patient and set realistic expectations. Offer patients a copy of the consent form to review at home with family members.2
___________________________
1 “Off-Label” and Investigational Use of Marketed Drugs, Biologics, and Medical Devices—Information Sheet. U.S. Food and Drug Administration. Last updated Jan. 25, 2016. Accessed Jan. 4, 2017.
2 For information on risk assessments and sample consent forms, see www.omic.com.
|
Meet the Experts
ALAA M. ELDANASOURY, MD Medical director and chief of the cornea and refractive surgery units at the Magrabi Eye and Ear Hospitals in Jeddah, Saudi Arabia; past president of the International Society of Refractive Surgery. Relevant financial disclosures: None.
BENNIE H. JENG, MD Professor and chair of ophthalmology and visual sciences at the University of Maryland School of Medicine in Baltimore. Relevant financial disclosures: None.
ANNE M. MENKE, RN, PHD Risk manager at the Ophthalmic Mutual Insurance Company (OMIC) in San Francisco. Relevant financial disclosures: OMIC: E.
J. BRADLEY RANDLEMAN, MD Professor of ophthalmology at Keck School of Medicine of the University of Southern California and director of cornea, external disease, and refractive surgery at USC’s Roski Eye Institute, both in Los Angeles; editor-in-chief of the Journal of Refractive Surgery. Relevant financial disclosures: None.
CHRISTOPHER J. RAPUANO, MD Director of the cornea service and co-director of the refractive surgery department at Wills Eye Hospital and professor of ophthalmology at Jefferson Medical College, both in Philadelphia. Relevant financial disclosures: OMIC: C.
For full disclosures and the disclosure key, see below.

|