Download PDF
Cyclodestructive procedures include a variety of modalities that are used to selectively destroy the ciliary body to reduce aqueous secretion and lower IOP. Although many methods have historically been used for cyclodestruction, contemporary approaches most commonly use a laser to perform cyclophotocoagulation (CPC), either transsclerally or endoscopically. Cyclodestructive procedures are typically reserved for patients with 1) refractory glaucoma who have inadequate IOP control despite maximal medical therapy and surgery or 2) patients with poor visual potential in whom the risks of incisional surgery outweigh the benefits. Recently, though, because of advances in cyclodestructive technology, some authors have advocated for the use of these procedures in less-advanced cases of glaucoma.
Indications
Glaucoma patients with elevated IOP who have poor visual acuity potential, as well as eyes that are not candidates for or have failed incisional glaucoma surgery are typically considered for cyclodestructive procedures. Another indication is for pain relief in eyes with poor or no vision.
Contraindications. Relative contraindications include uveitic glaucoma, as the procedure can exacerbate inflammation.
Other considerations. If a nonpenetrating form of cyclophotocoagulation is performed in the office, patient cooperation is essential.
Transscleral CPC
In transscleral CPC (TS-CPC), laser energy is delivered through the sclera to the ciliary body. The melanin in the ciliary processes absorbs the laser energy, which leads to coagulative necrosis of the ciliary body and reduction of aqueous secretion.
TS-CPC can be performed in the office, with use of local anesthesia, or in the operating room. Types of anesthesia include peribulbar, retrobulbar, subconjunctival, sub-Tenon, topical, and general. Typically, peribulbar or retrobulbar block is required for TS-CPC. The semiconductor diode laser has largely supplanted the Nd:YAG laser for TS-CPC because of its greater efficacy.
Noncontact Nd-YAG laser. At the slit lamp, with or without a contact lens, an Nd:YAG laser is aimed 1.0 to 1.5 mm posterior to the surgical limbus for 270 to 360 degrees, avoiding the 3- and 9-o’clock positions to prevent damage to the long ciliary nerves.
Contact Nd-YAG laser. The Nd:YAG laser is generated from a sapphire probe connected to a fiberoptic system and applied directly to the conjunctiva, about 0.5 to 1.0 mm posterior to the surgical limbus for 270 to 360 degrees, avoiding the 3- and 9-o’clock positions. Ocular transillumination may be performed to verify the position of the ciliary body. Proper orientation and angle in relation to the sclera are necessary to minimize collateral damage.
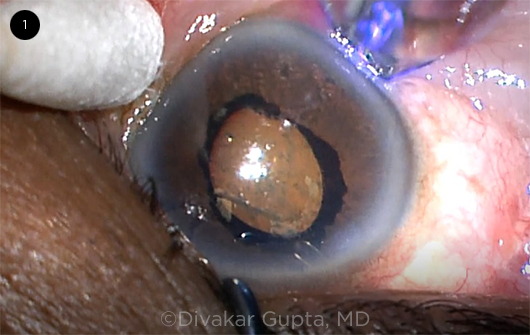
Semiconductor diode laser. CPC performed with a semiconductor diode laser is similar to the contact Nd:YAG technique but employs a probe emitting a continuous laser at 810 nm. It is positioned 1 to 2 mm posterior to the limbus and angled parallel to the visual axis for 270 to 360 degrees (Fig. 1), avoiding the 3- and 9-o’clock positions.
Standard parameters: 16 to 24 spots are typically applied, ranging from 1,250 to 2,500 mW, for a duration of 2,000 ms; energy is titrated to just below the level at which an audible pop is produced from destruction of the ciliary body.
An alternative protocol (slow coagulation technique) has different parameters: 3,500 to 4,500 ms duration with energy of 1,250 to 1,500 mW. This protocol has similar success rates as the standard protocol.1-4
Micropulse transscleral laser treatment (MP-TLT). While technically similar to the diode laser and also delivered through the sclera, MP-TLT differs in that it emits laser energy in repetitive quick pulses. This method minimizes collateral damage to tissue surrounding the pigmented ciliary epithelium by allowing periods of cooling between pulses.
In MP-TLT, a probe is placed perpendicularly at the limbus and moved back and forth in a continuous, sweeping motion (“painting”) for 180 to 360 degrees, avoiding the 3- and 9-o’clock positions. The duty cycle is set for 31.33% (on for 0.5 ms and off for 1.1 ms) at power of 2,000 mW for a duration of 100 to 360 seconds.1,2,5
In addition to selective destruction of the ciliary body, other hypothesized mechanisms of action include ciliary muscle contraction, allowing for posterior movement of the scleral spur and increased outflow through the trabecular meshwork; increased uveoscleral outflow has also been postulated. MP-TLT has gained some popularity for earlier use in glaucoma treatment, but data are limited on its efficacy or relative safety profile.
 |
|
TS-CPC. A semiconductor diode laser probe is placed 1 to 2 mm posterior to the limbus over the location of the ciliary body; the cotton-tipped applicator is used to help position the globe.
|
Endocyclophotocoagulation
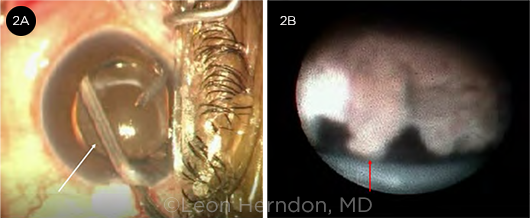
In the endocyclophotocoagulation (ECP) procedure, a fiberoptic intraocular endoscope with a semiconductor 810-nm diode laser is inserted via a limbal (most common) or pars plana approach. The endoscope is used to directly visualize and deliver laser energy to the ciliary body (Fig. 2). The ciliary processes are targeted and destroyed for 270 to 360 degrees (depending on the number of corneal incisions). The laser power is 150 to 300 mW, titrated until the processes blanch and shrink.
Unlike TS-CPC, this technique allows for controlled targeting of the ciliary processes without collateral damage to the ciliary muscle and associated stromal tissues, thus avoiding some of the associated side effects. However, ECP is a surgical procedure involving intraocular entry and, consequently, risk of infection. Notably, it has been combined successfully with cataract surgery and may be used in nonrefractory glaucoma cases.
 |
|
ECP. (2A) The white arrow points to the endoscope viewing the ciliary body posterior to the iris. (2B) View through the endoscope. The red arrow identifies one of the ciliary processes seen during ECP.
|
Other Techniques
High-intensity focused ultrasound achieves cyclodestruction through targeted beams of ultrasound energy delivered by piezoelectric transducers, resulting in targeted thermal necrosis of the ciliary epithelium with low risk of collateral damage. Results have shown efficacy and good outcomes, but this method is not available in the United States.
Transpupillary CPC is the application of an argon laser beam through the pupil to the ciliary processes. Because this method requires visualization of the ciliary processes, its use is very limited, restricted to patients whose pupils dilate well or those with aniridia.
Earlier types of cyclodestruction that are no longer commonly used include cyclocryotherapy (freezing of the ciliary body), cyclodiathermy (use of electrical currents to produce heat at the ciliary body), and cyclectomy (direct surgical removal of the ciliary body).
Complications
The most common potential complications of cyclophotocoagulation are hyphema, inflammation, cystoid macular edema, need for retreatment, and loss of vision. Hyphema is more common in eyes with neovascular glaucoma. Hypotony, sympathetic ophthalmia, and phthisis bulbi are rare but serious and feared conditions.
Vision loss is more common with TS-CPC than with ECP. One study found the incidence of hypotony to be 10% and that of sympathetic ophthalmia to be 0.07% with TS-CPC. ECP has the lowest risks of hypotony or phthisis bulbi but carries the risk of endophthalmitis. Of note, slow coagulation TS-CPC, compared with the standard protocol, was associated with shorter periods of inflammation and lower rates of pain and hyphema.6
Outcomes
Transscleral CPC and ECP are very effective in lowering IOP in refractory glaucoma and reducing the number of medications required. Although cyclodestructive procedures have often been pursued for refractory glaucoma, there is a dearth of literature comparing the efficacy of primary cyclodestructive procedures to other types of glaucoma surgery.
One randomized controlled trial that compared the efficacy of ECP to the Ahmed drainage implant for refractory glaucoma found no differences in visual acuity or IOP outcomes; however, the Ahmed implant had a higher incidence of complications.7 Another retrospective study looked at patients in whom primary drainage devices failed. The researchers compared outcomes between patients who received a subsequent glaucoma drainage device versus those who had TS-CPC and found that the TS-CPC group experienced greater reduction in IOP as well as fewer adverse events.8
Furthermore, little evidence is available to compare the different cyclodestructive procedures. One randomized controlled trial compared pulsed (MP-TLT) to continuous wave application for TS-CPC and found that although both were successful in lowering IOP, the MP-TLT procedure had more consistent results and fewer adverse events.9 A recent study that looked at the efficacy of TS-CPC in treating glaucomatous eyes without previous incisional ocular surgery found that TS-CPC was especially efficacious in eyes with IOP greater than 21 mm Hg that was refractory to maximal medical therapy; no severe or long-term complications were observed.10
Conclusion
Cyclodestructive procedures are efficacious procedures that can lower IOP. TS-CPC continues to be reserved for refractory glaucoma and those eyes with low visual potential.
Ongoing research is needed to evaluate newer techniques such as MP-TLT and the utility of cyclodestruction in eyes without refractory or end-stage glaucoma.
___________________________
1 Ndulue JK et al. J Ophthalmic Vis Res. 2018;13(1):55-61.
2 Anand N et al. Semin Ophthalmol. 2020;35(5-6):261-275.
3 Quigley HA. J Glaucoma. 2018;27(8):674-681.
4 Duerr ER et al. Ophthalmol Glaucoma. 2018;1(2):115-122.
5 Quigley HA. Ophthalmol Glaucoma. 2020;3(3):171-173.
6 Aujla JS et al. Clin Exp Ophthalmol. 2013;41(8):761-772.
7 Lima FE et al. J Glaucoma. 2004;13(3):233-237.
8 Levinson JD et al. J Glaucoma. 2017;26(4):311-314.
9 Aquino MC et al. Clin Exp Ophthalmol. 2015;43(1):40-46.
10 Sheheitli H et al. Ophthalmol Glaucoma. 2021;4(5):472-481.
___________________________
Dr. Gross and Dr. Aggarwal are second-year ophthalmology residents, and Dr. Gupta is an associate professor and glaucoma specialist; all are at the Duke University Eye Center in Durham, N.C. Financial disclosures: None.