By Lori Baker-Schena, EdD, MBA, Contributing Writer, interviewing John P. Berdahl, MD, Michelle R. Butler, MD, Cynthia Mattox, MD, and Thomas W. Samuelson, MD
Download PDF
Wednesday, Aug. 29, 2018, was “the day that rocked the glaucoma world,” as Thomas W. Samuelson, MD, describes it. That’s when Alcon urgently called for a “voluntary medical device market withdrawal” of its CyPass microstent, a minimally invasive glaucoma surgery (MIGS) device.
Two months later, the voluntary withdrawal was changed to an FDA Class 1 recall.
Unfamiliar Territory
CyPass was pulled from the market because of safety concerns based on five-year data from the COMPASS XT study, which indicated a higher rate of endothelial cell loss (ECL) in patients who underwent cataract surgery and received a CyPass than in those who had cataract surgery alone.
“In the field of ophthalmology, we had not experienced a device withdrawal like this before,” said Michelle R. Butler, MD, who practices in Dallas. And it stunned ophthalmologists who had experienced success with the device.
For instance, John P. Berdahl, MD, who practices in Sioux Falls, South Dakota, estimated that he had implanted more than 60 CyPass devices since the FDA’s initial approval, with good results. “I found that patients with moderate glaucoma and intraocular pressures [IOPs] on the lower side of the teens benefited from CyPass because we were able to create a conduit from the anterior chamber to the suprachoroidal space, allowing us to bypass Schlemm’s canal and drain the aqueous internally,” he said.
Initial upheaval, then guidelines. Cynthia Mattox, MD, president of the American Glaucoma Society, said her top priority was disseminating information to the members as quickly as possible. She stayed in touch with the Ophthalmic Mutual Insurance Company (OMIC) and Alcon, and she participated in conference calls with investigators.
Initially, the recall sparked considerable confusion among clinicians, especially those who had implanted the device in their patients, Dr. Mattox noted.
But in short order, the American Society of Cataract and Refractive Surgery (ASCRS), the FDA, and OMIC issued guidance for clinicians.1-3 That guidance continues to be updated as needed. (See “Where We Are Now.”)
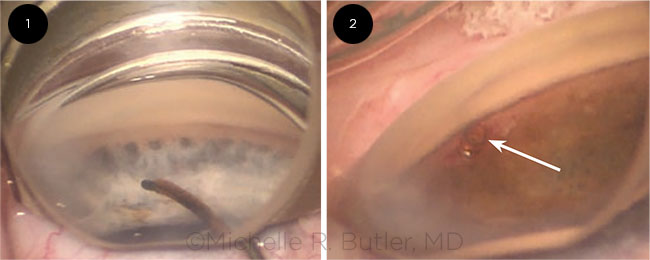 |
|
IN PLACE. The CyPass during surgery (Fig. 1) and after implantation (Fig. 2).
|
A CyPass Primer
CyPass is one of several MIGS devices developed to serve as a minimally invasive alternative to conventional glaucoma surgery. These newer surgical options can allow patients to reduce their medication burden and modestly improve overall pressure control while avoiding some of the complications associated with traditional glaucoma surgery, Dr. Butler said.
Given their favorable safety profile and modest efficacy, the various MIGS devices have gained popularity in the treatment of patients with mild to moderate glaucoma.
How it differs. “Many of the MIGS devices [and procedures] are Schlemm’s canal–based,” said Dr. Butler. “CyPass was the first MIGS device to use an entirely new outflow pathway,” giving clinicians another option for many patients.
Initial approval. CyPass received approval from the FDA on July 29, 2016, based on two-year safety and efficacy data from the COMPASS trial.4 In this study, 374 patients received the CyPass in combination with cataract surgery, and 131 patients underwent cataract surgery alone (controls). At 24 months, 77% of the microstent subjects achieved greater than 20% unmedicated IOP lowering versus 60% of control patients.
In addition, mean reduction in IOP was 7.4 mm Hg for the CyPass group versus 5.4 mm Hg in the control group, with 85% of microstent subjects not requiring IOP-lowering drugs at 24 months.
At this point, Dr. Butler said, the rate of ECL was 11.2% in the CyPass patients and 7.9% in controls.
Call for additional safety data. After the FDA approved CyPass, it mandated an additional three years of safety data be collected from these patients. Named the COMPASS XT trial, this study included 200 CyPass patients and 53 cataract controls.
Troubling results. The COMPASS XT study uncovered a significant difference in ECL between CyPass recipients and the control group at 48 and 60 months. At month 48, the CyPass group experienced an 18.4% rate of ECL, versus 7.5% in the control group. At the 60-month mark, those rates of loss were 20.4% in the CyPass group and 10.1% in the control group.
Pinpointing the problem. An ASCRS task force, which included Drs. Berdahl and Samuelson, wrote up a preliminary statement that provided both an analysis of the COMPASS XT results as well as recommendations for clinicians.
The task force noted a correlation between CyPass implantation depth and the rate of ECL, with the number of device rings visible used to grade implantation depth. ECL was 1.39% per year for eyes with no rings showing, 2.74% per year for eyes with one ring showing, and 6.96% per year for eyes with two to three rings showing.
Additional findings. On a positive note, no patients in COMPASS XT required corneal surgery during the five years of the trial. One case of corneal edema was documented; it resolved by the completion of the study.5
|
|
For more about Cypass, view an interview with Ruth D. Williams, MD, and Thomas W. Samuelson, MD.
|
Where We Are Now
Here is a brief compilation of guidance for clinicians, drawn from ASCRS, the FDA, and OMIC:
Product return. Return unused devices to Alcon.
Notify affected patients and conduct baseline exams. The clinician should promptly 1) notify patients who have received a CyPass that the device has been withdrawn and 2) conduct a baseline examination to document the device’s position and determine the patient’s risk.
Assess device positioning. ASCRS recommends documenting the presence or absence of contact between the corneal endothelium and the device, the position of the device lumen anterior to Schwalbe’s line, and the number of retention rings visible in the anterior chamber.
The FDA’s language is as follows: “Eye care providers should … assess device positioning by visualization of the number of retention rings visible on the proximal end of the device. Patients with two or more rings visible on examination should be evaluated for ECL as soon as possible.”2
Develop a monitoring plan. Without clear evidence of corneal decompensation, no action other than clinical monitoring is recommended in patients who have one ring (or no rings) of the CyPass visible in the anterior chamber by gonioscopy, the ASCRS task force said.
The task force report also pointed out that while there is a greater risk of corneal ECL in patients with two or three rings of the CyPass device visible in the anterior chamber by gonioscopy, “not all eyes will experience clinically meaningful ECL.” It added, “Without clinically significant evidence of corneal decompensation, no action other than monitoring is indicated.”
Specular Microscopy
Dr. Samuelson, president of ASCRS and in practice in the Minneapolis area, noted that the ASCRS guidelines recommend that specular microscopy be considered for those patients at increased risk (two or more rings visible or the lumen of CyPass is above Schwalbe’s line). In contrast, the FDA recommends using specular microscopy to evaluate all CyPass recipients until the rate of ECL stabilizes.
What if you don’t have a specular microscope? In its latest recommendations,3 OMIC suggests the following:
- Identify practices or academic centers where counts are available, and determine the cost of the count.
- If the exam does not indicate any relevant problems, tell the patient that you do not feel a count is needed at this time.
- At the same time, give the patient the option of having the count done, and explain the cost.
- Document all of the above.
What About Device Revisions?
What about patients with CyPass devices who fall into the high-risk category due to the number of retention rings visible on the proximal end of the device?
After the first few weeks of implantation, it is difficult to reposition or remove the device because of fibrosis, and such manipulation carries a risk of complications, Dr. Butler cautioned. For these high-risk patients, ASCRS recommends trimming the proximal end.
However, as Dr. Butler pointed out, relatively few surgeons have experience trimming a CyPass. Doing so requires a bimanual technique to stabilize and trim the stent and additional help to hold the gonioprism in place for visualization. She added that the decision to trim a high-risk stent must be weighed against the risk of the procedure itself.
For its part, the FDA says, “Based on the endothelial cell density levels, and other factors such as age and time postimplantation, the surgeon should determine if additional surgical interventions (that is, trimming, repositioning, or removal) are appropriate.”
Weighing the Risks
“Perspective is important” when considering the ramifications of the withdrawal, Dr. Samuelson said. “Interestingly, none of our traditional glaucoma devices [or procedures]—such as long tubes or trabeculectomy—has been held to a safety standard that compares the procedure to the safety of cataract surgery alone. That is a high bar.”
“It is important to keep things in perspective,” Dr. Mattox agreed. “Glaucoma is a long war—and [with] each of these little battles you fight, you may not win everything, but you need to keep fighting for the patient’s vision.” Dr. Mattox added, “We don’t want to see so much reaction to this recall that we stifle the whole field. Ultimately, with iterations and proper risk management, both patients and doctors will benefit from new technology. However, it is important that we remain transparent so we can learn and optimize the options for patients.”
Patients with moderate or more severe disease who have received a CyPass might possibly still benefit from the device, Dr. Samuelson said. And with regard to evaluating risk, he added that there are other more traditional procedures to treat glaucoma, “yet those may pose at least as much—if not more—risk in terms of endothelial cell loss. Interestingly, this has not been studied.”
Dr. Samuelson also cited an article6 that asserted that damage to the corneal endothelium may actually be caused by the glaucoma disease process itself as well as by treatment alternatives. However, none of the existing glaucoma surgical devices has five-year ECL data to serve as a comparator to data generated by the COMPASS XT study. The authors called for more research on ECL as it relates to both glaucoma and its various treatments.
Confidence in the Process
Dr. Berdahl noted that he has not “given up hope” that CyPass could be reintroduced to the marketplace. “Upon analyzing the [COMPASS XT] data, I wasn’t worried from a patient standpoint. My read on the data was that there is a significant loss of endothelial cells in the CyPass patients, but it hasn’t turned into a clinical problem. Alcon acted on the side of caution.”
Looking back over the CyPass recall, Drs. Berdahl and Samuelson praised the cooperation that took place among physicians and organizations as the recall rolled out.
“The process worked well in this scenario,” Dr. Berdahl said. “The FDA approved the device while mandating extension studies. A negative safety signal was noted, and the device was withdrawn from the market.”
For his part, Dr. Samuelson concluded, the system “is working just like it was intended [to]. Based on subclinical findings, Alcon was able to act swiftly and effectively to stop implantation. The FDA has cast a big safety net—all before any clinical signs [of harm were identified]. Now we are in this regroup mode trying to identify why patients are losing cells.”
___________________________
For a discussion of CyPass positioning and the need to monitor patients, see aao.org/interview/cypass-withdrawal-from-cornea-surgeon-s-perspectiv.
___________________________
1 http://ascrs.org/sites/default/files/Preliminary_ASCRS%20_yPass_Withdrawal_Consensus_Statement.pdf. Accessed Dec. 5, 2018.
2 www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm624283.htm. Accessed Dec. 5, 2018.
3 www.omic.com/fda-recalls-cypass-glaucoma-device. Accessed Dec. 17, 2018.
4 Vold S et al. Ophthalmology. 2016;123(10):2103-2112.
5 www.alcon.com/content/cypass-micro-stent-market-withdrawal. Accessed Dec. 5, 2018.
6 Janson BJ et al. Surv Ophthalmol. 2018;63(4):500-506.
___________________________
Dr. Berdahl is a glaucoma and cornea specialist at Vance Thompson Vision in Sioux Falls, S.D. Relevant financial disclosures: Alcon: C,L; Allergan: C,L; Glaukos: C,O.
Dr. Butler is a glaucoma specialist with Glaucoma Associates of Texas and assistant professor of ophthalmology at UT Southwestern Medical Center, both in Dallas. Relevant financial disclosures: Allergan: L.
Dr. Mattox is a retired glaucoma specialist and current president of the American Glaucoma Society. Relevant financial disclosures: Alcon: C,S; Allergan: C,S; Ivantis: C; New World Medical: C; Santen: C.
Dr. Samuelson is a glaucoma/anterior segment specialist, founding partner of Minnesota Eye Consultants, and current president of ASCRS. Relevant financial disclosures: Alcon: C; Glaukos: C,O; Ivantis: C,O; Santen: C; Sight Sciences: C.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Berdahl Alcon: C,L; Allergan: C,L; AMO: C; Aurea: C,O; Avedro: C; Bausch + Lomb: C; Calhoun Vision: C; ClarVista: C; DigiSight: C,O; Envisia: C; Equinox: C,O; Glaukos: C,O; Imprimis: C,P; Ocular Surgical Data: C,O; Ocular Therapeutix: C; Omega Ophthalmics: C,O; Oyster Point: C,O; Vittamed: C.
Dr. Butler Allergan: L.
Dr. Mattox Aerie: C; Alcon: C,S; Allergan: C,S; Ivantis: C; New World Medical: C; Novartis: C,S; Ocular Therapeutix: C; Santen: C.
Dr. Samuleson Aerie: C; Akorn: C; Alcon: C; AMO: C; AqueSys/Allergan: L; Bausch + Lomb: C; Belkin Laser: C; Equinox: C,O; Glaukos: C,O; Ivantis: C,O; Ocular Surgery News: C; Santen: C; Shire: C; Sight Sciences: C; Transcend Medical: C; Slack: C.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|