By Norberto Mancera, MD, Gonzalo Ortiz, MS, Hershel Patel, MD, and Vignesh Raja, MD
Edited by Steven J. Gedde, MD
Download PDF
At first, Patrick Williams* was bothered by an occipital headache, which dragged on for 2 to 3 months. But it was the sudden onset of binocular horizontal diplopia that finally prompted the 71-year-old to seek medical treatment.
We Get a Look
Mr. Williams had a history of type 2 diabetes and hypertension, but he told us that he had not experienced any previous episodes of diplopia. He added that his horizontal diplopia was greater at distance than at near—and that it was worse with right gaze.
During our examination, we found his visual acuity to be 20/20 in both eyes. His pupils were equally round and reactive with no afferent pupillary defect, and the slit-lamp exam was unremarkable. During extraocular muscle testing, we observed an isolated right sixth nerve palsy, with inability to abduct his right eye on right lateral gaze. His left eye had fully intact extraocular muscle movement, and we observed no other neurological or systemic symptoms. Given Mr. Williams’ history of type 2 diabetes and hypertension and his current clinical presentation, we suspected that he had a microvascular right sixth nerve palsy.
We decided to monitor Mr. Williams closely, and follow-up appointments were scheduled. One month later, his condition had improved—and at the 3-month appointment, he reported complete resolution of both his headache and palsy.
Differential Diagnosis
The differential diagnosis for any lateral rectus palsy is quite broad, and the clinician should consider the presenting symptoms in the context of underlying medical disease, trauma, birth history, age, sex, and risk of infections.
A limited differential includes microvascular palsy, metastatic disease, Duane syndrome, orbital fracture, giant cell arteritis, tumor, aneurysm, cavernous sinus thrombosis, Lyme disease, multiple sclerosis, and myasthenia gravis.
The most common cause of sixth nerve palsy is believed to be a microvascular insult, and vasculopathic risk factors include diabetes, hypertension, and hypercholesterolemia. A patient’s history of coronary artery disease, myocardial infarction, stroke, and smoking should be considered.1
The classic approach when encountering a patient with these risk factors and a sixth nerve palsy is to observe for resolution. Further workup may be pursued if symptoms don’t resolve or worsen in 3 months. Thus, Mr. Williams was slated for observation, as his risk factors and presentation appeared to be attributable to a vasculopathy, especially given his history of diabetes and hypertension.
An Unexpected Return
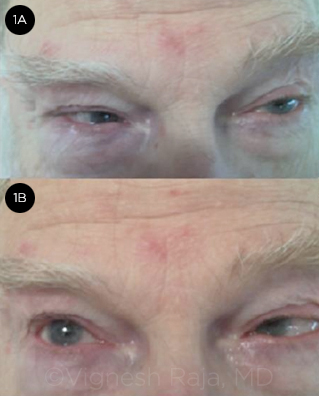
Unfortunately, the story did not end there. Mr. Williams returned 11 months later. At this point, he was exhibiting a bilateral sixth nerve palsy (Fig. 1). Conservative follow-up was no longer an option, and given Mr. Williams’ bilateral presentation and age, we decided to pursue imaging studies.
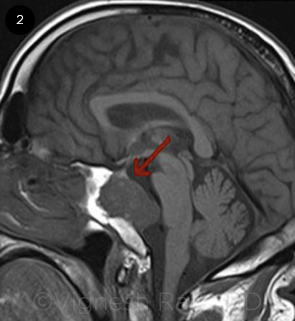
Magnetic resonance imaging (MRI) revealed a chordoma in the skull base, centered on the clivus and compressing the pons, brainstem, and cerebellum (Fig. 2, arrow). Mr. Williams subsequently underwent transsphenoidal debulking of the chordoma.
 |
|
PROGRESSION. When the patient returned, his sixth neve palsy was bilateral, with lack of abduction in both eyes.
|
Discussion
A chordoma is an uncommon neoplasm of notochordal origin that is both slow growing and locally aggressive. The 2 most common areas of occurrence are at the clivus and the sacrococcygeal spine. The prognosis for clival chordoma is poor because of its proximity to delicate neurovascular structures and its tendency to recur.
Presentation. Chordomas most commonly occur in the fourth and fifth decades of life, and two-thirds of those affected are men. The most common clinical presentation includes symptoms of headache and cranial nerve palsies. In one study of 48 patients with chordoma, 16 developed diplopia as their first symptom, 17 initially had headache, and 8 experienced both symptoms simultaneously. Only 1 patient initially complained of decreased visual acuity.2
The most prevalent deficit tends to be an isolated left lateral rectus palsy, followed by bilateral lateral rectus involvement. Isolated right lateral rectus involvement is considered the least common presentation.3
Kline and Glaser reported a patient who initially presented with bilateral sixth nerve palsy. As in our case, their patient lacked other neurological findings and was older than is typically associated with clival chordoma.4
Workup. The clinician should consider a workup with imaging and/or lumbar puncture when encountering a patient with recurrent or bilateral sixth nerve palsy, even if the patient has a known microvascular disease such as diabetes or hypertension. It is also important to differentiate chordoma from chondrosarcoma, as both are locally invasive skull base tumors. Although the 2 have similar presentations in terms of clinical symptoms as well as in their imaging features,5 the characteristics of visual loss, facial numbness, and multiple cranial neuropathies are more common in patients with chondrosarcoma.2
Imaging. In the past, computed tomography was the preferred method of imaging, but MRI and angiography have become valuable in delineating the anatomy of soft tissues. A clival chordoma is often difficult to diagnose; it has been shown that an off-midline growth pattern is indicative of a chondrosarcoma and helps in differentiating it from a chordoma on conventional MRI in the majority of patients.5 Additionally, diffusion-weighted MRI may be a promising tool for distinguishing between these particular skull base tumors.5
Treatment. Given clival chordoma’s high rate of recurrence, the treatment of choice remains gross total resection (GTR) of the tumor with postoperative radiation therapy. However, the chordoma may still recur after GTR. In a recent retrospective review, the lower third of the clivus often contained residual or recurrent tumor despite staged approaches that provided mediolateral (transcranial + endonasal) or superoinferior (endonasal + transoral) access. No additional benefits were observed with proton-based or photon-based radiation.6 Success of GTR depends on the tumor’s size, location, and proximity to vital structures.
As researchers work to characterize chordoma cell lines, future treatment options may include targeted chemotherapeutic agents. In addition, expression of the Brachyury gene, a transcription factor in the notochord, may prove to be a diagnostic marker for chordoma.6 As the field of molecular medicine expands, bench to bedside innovations may aid in the diagnosis and management of chordomas.
 |
|
NEOPLASM. MRI revealed a clival chordoma in the skull base (arrow).
|
Conclusion
Clival chordomas are rare intracranial tumors. It is important to pay close attention to the neuro-ophthalmological exam, as patients with these masses often present with ophthalmic signs first, typically with dysfunction in cranial nerves involved in ocular movement. In our case, right sixth nerve palsy was the primary presenting symptom in a patient with a clival chordoma. His condition spontaneously resolved in the manner of a microvascular sixth nerve palsy, but he later developed a bilateral palsy. Both the physical exam and imaging studies were integral in properly addressing his condition.
___________________________
* Patient name is fictitious.
___________________________
1 Tamhankar MA et al. Ophthalmology. 2013;120(11):2264-2269.
2 Volpe NJ et al. Am J Ophthalmol. 1993;115(1):97-104.
3 Harbour JW et al. Skull Base Surg. 1991;1(4):200-206.
4 Kline LB, Glaser JS. Ann Ophthalmol. 1981;13(6):705-707.
5 Muller U et al. Acta Radiol. 2016;57(2):225-232.
6 Jahangiri A et al. Neurosurgery. 2015;76(2):179-185.
___________________________
Dr. Patel is a second-year ophthalmology resident, Dr. Mancera is a first-year ophthalmology resident, and Mr. Ortiz is a fourth-year medical student; all 3 are at the University of South Florida in Tampa. Dr. Raja is a consultant ophthalmologist and head of the Department of Ophthalmology at Sir Charles Gairdner Hospital in Perth, Western Australia. Relevant financial disclosures: None.
More at the Meeting
Neuroimaging in Ophthalmology (Lab117). When: Sunday, noon-2:00 p.m. Where: Room N231. Access: Ticket required.
Diagnostic and Therapeutic Dilemmas in Neuro-ophthalmology (252). When: Sunday, 2:00-4:15 p.m. Where: Room E352. Access: Academy Plus course pass.
Evaluating an Adult With Acute Diplopia (B171). When: Tuesday, 7:30-8:30 a.m. Where: Hall A. Access: Ticket required.
|