Download PDF
Anti-VEGF therapy has been a godsend for many patients with diabetic macular edema. But it’s not the whole answer for all patients. Greater personalization is still needed.
Many studies support the view that the natural history of diabetic macular edema [DME] moves from being a permeability-based disease, in which patients respond very well to anti-VEGF monotherapy, to a multifactorial disease that is inflammation based,” said Pravin U. Dugel, MD, at Retinal Consultants of Arizona in Phoenix and USC Eye Institute, Keck School of Medicine in Los Angeles.
One problem is that the nomenclature hasn’t yet caught up. For example, he said, “Oncologists benefit from the specificity of a stage 2A breast cancer diagnosis, which informs a woman’s prognosis and treatment. But ophthalmologists simply lump all cases of DME into one group.”
When it comes to DME treatment, one size certainly doesn’t fit all, added David S. Boyer, MD, with the Retina-Vitreous Associates Medical Group in Los Angeles. “Personalized treatment involves titrating based on what we think is causing most of the problem. Some [cases] are purely VEGF driven, some are a combination of VEGF and inflammatory driven, and some are mainly inflammatory driven.”
Here, 5 retina experts offer their insights about managing this complex condition, describing the strengths and limits of current types of treatment as well as potential targets for novel approaches on the horizon.
 |
|
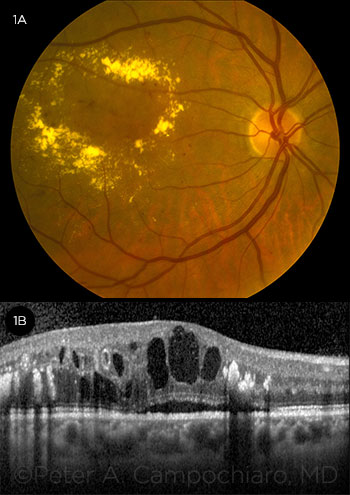
DIABETIC MACULAR EDEMA. (1A) fundus photograph and (1B) OCT image.
|
Paradigm Shift in Treatment
Just 5 years ago, DME was mainly treated by focal or grid laser photocoagulation, said Lloyd Paul Aiello, MD, PhD, at Harvard Medical School, Boston. For nearly 2 decades, that was the paradigm for DME treatment. “Its primary benefit was to reduce the risk of worsening vision by about half.”
Limits of laser. However, laser had its limitations, including scarring and scotomata, said Dr. Dugel. In addition, it could not be used in centrally involved DME because the laser would impair central vision.
For reasons like these, focal laser has become less of a first-line therapy and more of a niche treatment, said Julia A. Haller, MD, at Wills Eye Hospital in Philadelphia. “It’s used most often for patients with very focal areas of edema such as those with circinate rings of lipid where the leakage is from one focal spot,” she said, “and as an adjunct to pharmacologic treatment that isn’t working well or wears off quickly.”
Shift to anti-VEGF therapy. Today, we have clear evidence that VEGF plays an important role in the development of DME, said Peter A. Campochiaro, MD, at Johns Hopkins Medicine in Baltimore. “Studies have demonstrated that many patients benefit substantially from suppression of VEGF.”
With the advent of anti-VEGF drugs, the paradigm shifted very dramatically, said Dr. Aiello. “These drugs could not only prevent vision from getting worse but could also recover substantial amounts of vision—nearly doubling the improvement you could get compared with laser and cutting the risk of getting worse by more than two-thirds.”
Anti-VEGF therapy has increasingly become the first-line therapy, said Dr. Haller, not just for DME but for diabetic retinopathy (DR) as well. In fact, the Academy’s recently updated Preferred Practice Pattern: Diabetic Retinopathy states, “Intravitreal injections of anti-VEGF agents have been shown to be an effective treatment for center-involving diabetic macular edema and also as an alternative therapy for proliferative diabetic retinopathy.”1
“Contrary to the wait-until-very-clinically-significant treatment approach used previously with photocoagulation,” Dr. Haller said, “today we’re moving toward earlier treatment to hold on to better vision with DME and steer DR in a better direction, too.”
In contrast to age-related macular degeneration (AMD), said Dr. Aiello, it’s possible to maintain visual gains with DME using fewer and fewer anti-VEGF injections over time—on average, 6-8 the first year, 2-3 the second year, 1-2 the third year, and 0-1 the fourth and fifth years.
Limits of anti-VEGF therapy. “Although the number of injections does go down, patients still need to be seen on a regular basis to get optimal results. The number of injections is not a proxy for treatment burden,” said Dr. Dugel.
Another critical limitation is that not all patients have a full response to anti-VEGF therapy, said Dr. Aiello. “More than 80% will have some response, but about half [of those] will not have a complete response, where their vision and retinal swelling return to normal.”
Some patients on anti-VEGF agents get 12 letters of improvement and do well, added Dr. Boyer. “Another group of patients gets only 5 or 6 letters of improvement, despite continuing injections. A third group doesn’t respond well at all, indicating that VEGF is not the mechanism in those people. Patients tend to fall into—and stay in—these ‘swimming lanes.’ That gives us an opportunity to individualize treatment—to find out which patients can benefit the most from anti-VEGF therapy and which need another approach to get the best results visually.”
The EARLY Analysis Study. With this in mind, Dr. Dugel and colleagues wanted to see whether 3 monthly anti-VEGF injections could predict how patients would respond to treatment over time. “We found we could do that to a high degree of certainty, not only for 1 year, but for 3.” Dr. Dugel reported the study results during Retina Subspecialty Day 2016 in Las Vegas.
Methods. Dr. Dugel and his team conducted a post hoc analysis of raw data from the DRCR.net Protocol I study. They categorized 340 eyes by BCVA response (<5, 5-9, ≥10 letter improvement) after 3 monthly injections at 12 weeks.
Results. Patients with a strong response after 3 injections (≥10 letters at week 12) maintained this response over 3 years. Those with limited improvements (<5-9 letter gain) after 3 injections continued to see limited improvement during the entire study. And patients with less than 5 letter improvement early received less total benefit over the course of the study.
Exceptions. Nearly 40% of patients gained less than 5 letters of vision at 12 weeks. The researchers studied every one of these patients to see how many would improve with continued injections. “Twenty-eight percent did improve,” said Dr. Dugel, “but you have to ask, at what cost to the 72% who might continue receiving injections that are ineffective?” In studies such as RIDE and RISE, said Dr. Dugel, patients never caught up when given appropriate, yet delayed, treatment. “By delaying appropriate treatment, it would seem we leave some vision on the table.”
Personalized treatment. Results from the EARLY trial reflect what we see clinically, said Dr. Dugel, which is that we have to personalize treatment based upon response and that response can be identified as early as 12 weeks. “We don’t yet have a biomarker, test, or genetic analysis, and these patients don’t come with any other clues,” he said. “However, using 3 injections is a way to test whether a patient is in the permeability or inflammation phase of the condition.”
These findings will be important if they hold up, said Dr. Aiello. “However, it’s difficult to comment without yet seeing the published results.”
Robust Biomarker?
Many researchers are working to find a biomarker that can help predict VA and tell who will respond well to treatment and for how long, said Dr. Aiello.
He and coinvestigators have explored whether spectral domain optical coherence tomography (SD-OCT) parameters are correlated with VA in eyes with current or resolved center-involved DME. They evaluated images for disorganization of retinal inner layers (DRIL) and for cysts, epiretinal membranes, microaneurysms, subretinal fluid, and outer layer disruption/reflectivity.1 “Using OCT to look at the inner layers of the retina,” said Dr. Aiello, “we found that DRIL was highly correlated with both current and future vision loss. Early changes were highly predictive of vision changes 8 to 12 months later.” DRIL above a certain level was rarely associated with good vision but was commonly associated with bad vision. In addition, when DRIL improved, vision did as well.
Dr. Aiello and colleagues are conducting more studies to see if these results hold true with larger populations. At this point, though, DRIL is one of the very few markers that is highly correlated with vision and also predictive of future outcome, he said, unlike central retinal thickness, which explains no more than 27% of variation in VA.2
___________________________
1 Sun JK et al. Diabetes. 2015;64(7):2560-2570.
2 Browning DJ et al; Diabetic Retinopathy Clinical Research Network. Ophthalmology. 2007;114(3):525-536.
|
Individualizing DME Treatment
While ophthalmologists may lack diagnostic specificity for DME, personalization of treatment has already begun.
Start with anti-VEGF. “We don’t yet have good markers for who is going to be a good anti-VEGF responder and who is not,” said Dr. Aiello, “so we start with anti-VEGF therapy because VEGF is a major factor. Depending upon the response, we ask, ‘Does the patient require steroids or combination therapy, or is there another completely different molecule with a VEGF-independent pathway that may work—individually or in combination—for a more robust response?’” A number of phase 1 and phase 2 studies are currently looking at these, he added.
When to try steroids. Steroids can have a beneficial effect on macular edema equivalent to that of anti-VEGF drugs, said Dr. Aiello, at least in the short term. However, added Dr. Haller, steroids are mainly used only when anti-VEGF treatment either dries out the macula inadequately or wears off too quickly, or when frequent injections are prohibitive for the patient.
Limits of steroids. There are some concerns about the long-term effectiveness of steroids, said Dr. Aiello, but the big problem is the risk of cataract and elevated intraocular pressure (IOP). “At least 8 in 10 patients will eventually develop cataract, which is a significant confounder, making it hard to evaluate vision,” he said.
For a young patient with a clear lens, added Dr. Campochiaro, it’s preferable to “push” the anti-VEGF drug as much as possible before considering steroids.
Which steroid to pick. Three different types of steroids are available; 2 are FDA-approved implants. “To me, the choice is based entirely on pharmacokinetics [PK],” said Dr. Dugel, who described the 3 options.
- A bolus intravitreal injection (triamcinolone) is the cheapest, but it also has a PK profile with a quick rise and quick decrease. “This PK profile would be the least effective and also lead to the most side effects,” said Dr. Dugel.
- The Ozurdex (dexamethasone) implant is entirely biodegradable. “It provides an initial burst, with a gradual decline,” he said, “and is effective for 3 to 4 months.”
- The Iluvien (fluocinolone) implant, which is not biodegradable, provides steady state delivery of the drug and lasts up to 3 years.
Dr. Dugel first uses Ozurdex and watches to see if the drug arrests the problem after several treatments. “For patients who require longer-term suppression, I then switch to Iluvien,” he said. Using Ozurdex first helps fulfill an Iluvien label requirement, which is to demonstrate that the patient hasn’t previously experienced increased IOP from steroids.
Decision points. Dr. Boyer described the decision process he uses to individualize treatment, beginning at 4 weeks. “I begin all patients on Avastin [bevacizumab],” he said. “If there’s improvement at 4 weeks—the patient is partly or completely dry—I continue to treat with Avastin. If there’s only mild improvement, I might give another injection of Avastin or switch to Eylea [aflibercept]. If the patient is completely unresponsive to Avastin therapy after the first treatment—has poor vision and no decrease in edema—I will probably switch to Eylea. However, if there’s no response after 3 or 4 anti-VEGF treatments, it’s very likely the patient will improve with the addition of steroids.” If the patient does not improve significantly with the addition of steroids, Dr. Boyer then considers combination therapy with anti- VEGF, steroids, and laser.
 |
|
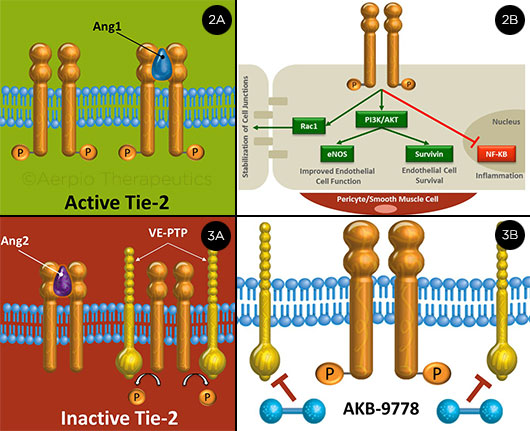
TIE-2: ACTIVE AND INACTIVE. (2A) Ang-1 activates Tie-2; (2B) this triggers multiple downstream vascular stabilizing signals. (3A) Ang-2 causes inactivation, as does VE-PTP, which dephosphorylates Tie-2. (3B) AKB-9778 inhibits VE-PTP.
|
New Pathways and Novel Approaches
Researchers are investigating other therapeutic targets.
Tie-2. “One of the other signaling pathways that seems to be important in DME is the Tie-2 pathway, which is related to a receptor on endothelial cells,” said Dr. Campochiaro. When stimulated, this receptor “produces reinforcement of junctional proteins, more interaction with surrounding cells and matrix, and stabilization of the vasculature, so there’s less leakage.” Following are some of the possible treatments that are being studied.
Block Ang-2. Angiopoietin-1 (Ang-1) is an endogenous protein that can activate Tie-2 to stabilize the vasculature (Figs. 2A, 2B). By contrast, Ang-2 competes for binding with Ang-1 and is high in patients with DME (Fig. 3A). One potential treatment approach, said Dr. Campochiaro, is to block Ang-2 with antibodies, thus allowing endogenous Ang-1 to activate Tie-2. A Regeneron trial will soon be launched to compare Eylea alone with Eylea plus an antibody to Ang-2 in patients with DME. Roche-Genentech is also testing an Ang-2 biphasic antibody to stabilize the membranes, said Dr. Dugel.
Directly activate Tie-2. Normally, a vascular endothelial-protein tyrosine phosphatase (VE-PTP) dephosphorylates Tie-2, keeping it in an inactivated state, said Dr. Campochiaro. When ischemia is present, VE-PTP is upregulated—and, therefore, is even more active in the setting of DME. Aerpio Therapeutics recently completed the TIME-2 study, a phase 2a study of a small molecule (AKB-9778) that inhibits VE-PTP and directly activates Tie-2 (Fig. 3B) (ClinicalTrials.gov; NCT02050828).
“We found that a combination of the AKB-9778 plus ranibizumab is superior to ranibizumab alone in reducing DME over 3 months,” said Dr. Campochiaro, an investigator in the trial. In addition, preliminary data suggest that activation of Tie-2 with AKB-9778 may cause improvement in DR. “AKB-9778 is relatively safe and can be given by self-administered subcutaneous injection—a technique that DME patients are familiar with.”
Niche for Tie-2? If Tie-2 agents are approved, Dr. Campochiaro envisions them being added for patients who don’t respond optimally to anti-VEGF therapy within a reasonable period of time—possibly after 3 to 6 injections. “If successful, these agents may help reduce the number of intraocular injections needed,” he said. “However, if future studies confirm that AKB-9778 improves diabetic retinopathy, it’s conceivable it could be used in many more patients with diabetic retinopathy whether or not they have DME.”
Permeability genes. Steroids downregulate a number of genes involved in permeability, said Dr. Campochiaro. “We know they affect multiple things, but we don’t really know which ones are critical, so this is a little like a black box.”
To learn more, Dr. Campochiaro and coinvestigators have measured levels of active proteins in the aqueous of DME patients and then administered Ozurdex and continued to take measurements over time, correlating these measurements with changes in macular edema. “This helps determine which proteins increase and which decrease with changes in edema, and may provide a number of candidates for potential treatment.”
Other novel options. A wide range of other studies are in the works, said Dr. Haller, including a phase 1 study of intravitreal infliximab, which is a monoclonal antibody against tumor necrosis factor α (TNF-α).
In addition, she said, the plasma kallikrein-kinin system is a key player in inflammatory processes and is implicated as a contributor in the pathogenesis of DME; therefore, a kallikrein-kinin inhibitor is being explored for its therapeutic efficacy in an upcoming phase 2 trial.2
Dr. Boyer is a clinical investigator in studies exploring 3 other novel treatments. Teprotumumab (River Vision Development) is an insulinlike growth factor 1 receptor (IGF-1) antagonist that appears to downregulate edema.
Luminate (Allegro Ophthalmics), previously known as ALG-1001, is an integrin peptide therapy that targets receptors involved in antiangiogenesis and vitreolysis.
And Optina (danazol; Ampio Pharmaceuticals) is a low-dose oral steroid that reduces leakage.
___________________________
1 American Academy of Ophthalmology, Retina/Vitreous Panel. Preferred Practice Pattern Guidelines: Diabetic Retinopathy. Updated January 2016. www.aao.org/ppp. Accessed Feb. 25, 2016.
2 Kita T et al. Diabetes. 2015;64(10):3588-3599.
DRCR.net: Refining Anti-VEGF Treatment Protocols
Treatment of patients with DME is evolving, said Dr. Aiello, inaugural chair of the Diabetic Retinopathy Clinical Research Network (DRCR.net), which has played a major role in helping to refine treatment protocols. “We know we can have major improvements in many patients if they are treated aggressively and at the right time, but this is an active area, and we’re likely to see more refinements in how to use both anti-VEGF therapy and alternatives.”
Dosing is one area in which some clarity has been achieved. “We thought that poor responses might be due to not neutralizing all the VEGF,” said Dr. Campochiaro. The data thus far suggest that’s not the case. “When you get to a dose of 0.3 mg of ranibizumab, you’ve achieved sufficient neutralization, and higher doses don’t produce a substantially better effect.”
Protocol V. This study is enrolled and ongoing, and it is examining the best time to start anti-VEGF, said Dr. Aiello. “Do you start with patients who have good vision—20/20 or better—or can you wait until it’s 20/32 or worse, which is the current guideline?” Primary results are expected in about 2 years, he said.
Three other recent DRCR.net studies have reported results.
Protocol I. At 5-year follow-up, substantial reduction in macular thickness was demonstrated in all 3 Protocol I treatment approaches for center-involving DME (ranibizumab plus prompt or deferred laser, laser with deferred ranibizumab, and triamcinolone plus laser and deferred ranibizumab). Patients who received initial ranibizumab therapy were likely to have better long-term improvement in visual acuity (VA) than those in the other 2 groups—a difference that was observed throughout the 5-year follow-up period.1
Protocol T. This study was a head-to-head comparison of 3 anti-VEGF drugs: aflibercept, bevacizumab, and ranibizumab. At year 2, all drugs improved edema and vision. However, in eyes where baseline vision was 20/50 or worse, aflibercept provided significantly more benefit than bevacizumab (p = .02) but not more than ranibizumab (p = .18).2 Dr. Campochiaro explains these data to his patients and lets them decide which agent to use. “Unless there are extenuating circumstances, such as a large copay or no insurance, most choose aflibercept or ranibizumab.”
Dr. Dugel emphasized, however, that this is not a switch study—it doesn’t provide evidence that starting with one drug and switching to another provides any benefit.
Protocol S. Although designed to look specifically at anti-VEGF treatment for proliferative DR, the Protocol S trial also included DME results. It found that anti-VEGF therapy is not inferior to panretinal photocoagulation for DR, and, in fact, provides a greater benefit for DME as well as for DR.3 “VEGF is involved not only in the later stages of DR, such as macular edema and neovascularization,” said Dr. Campochiaro, “but also in the progression of DR and capillary closure that drives the whole process.” This raises the question about how early to start treatment. “It’s unlikely we will do monthly injections just to treat the retinopathy in people with good vision,” he said, “but this would be a reasonable target if technologies are developed that provide sustained delivery of anti-VEGF drugs—whether through implants, gene transfer, or other methods.”
___________________________
1 Bressler SB et al; Diabetic Retinopathy Clinical Research Network. Am J Ophthalmol. 2016;164:57-68.
2 Wells JA et al; Diabetic Retinopathy Clinical Research Network. Ophthalmology. Published online Feb. 27, 2016.
3 Gross JG et al; Diabetic Retinopathy Clinical Research Network. JAMA. 2015;314(20):2137-2146.
|
Meet the Experts
LLOYD PAUL AIELLO, MD, PHD Professor of ophthalmology, vice chair for Centers of Excellence, and codirector of Diabetic Eye Disease Center of Excellence, Harvard Medical School; associate chief, Massachusetts Eye and Ear Infirmary, and medical director, Massachusetts Eye and Ear Joslin Diabetes Center, Boston. Financial disclosures: Eli Lilly: C; Genzyme: C; KalVista: C.
DAVID S. BOYER, MD Ophthalmologist with the Retina-Vitreous Associates Medical Group in Los Angeles. Financial disclosures: Aerpio: C; Alcon: C,L; Alimera Sciences: C; Allegro: C,O; Allergan: C,L; Bausch + Lomb: C; Bayer Healthcare Pharmaceuticals: C; Genentech: C,L; GSK: C; Merck: C; Neurotech: C,O; NotalVision: C; Ohr: C,O; Pfizer: C; Quantel Medical: C; Regeneron: C; Santen: C.
PETER A. CAMPOCHIARO, MD Professor of ophthalmology and neuroscience, Wilmer Eye Institute at Johns Hopkins Medicine, Baltimore. Financial disclosures: AbbVie: S; Advanced Cell Technology: C; Aerpio Therapeutics: C,S; Akebia: C; Alimera Sciences: C; Allegro: C,O; Allergan: S; Applied Genetic Technologies: C; AsclipiX: C; Eleven Biotherapeutics: C; Genentech/Roche: C,S; Genzyme: S; GlaxoSmithKline: S; Graybug: P,O; Intrexon: C; Kala: C; Oxford BioMedica: S; Regeneron: C,S; RegenX: C,S; Roche: S,C; Rxi: C,S.
PRAVIN U. DUGEL, MD Managing partner at Retinal Consultants of Arizona and the Retinal Research Institute in Phoenix; clinical professor of ophthalmology at the University of Southern California USC Eye Institute and Keck School of Medicine in Los Angeles. Financial disclosures: Abbott Medical Optics: C; Acucela: C; Aerpio Therapeutics: C,O; Alcon: C; Alimera Sciences: C,O; Allergan: C; Digisight: O; Genentech: C; Novartis: C; Ophthotech: C,O; Ora: C; Regeneron: C; ThromboGenics: C.
JULIA A. HALLER, MD Ophthalmologist-in-chief, Wills Eye Hospital; professor and chair of ophthalmology at Jefferson Medical College of Thomas Jefferson University and Thomas Jefferson University Hospitals; and codirector of the Wills Vision Research Center at Jefferson, Philadelphia. Financial disclosures: Advanced Cell Technology: C; Alcon: C; Allergan: C; Celgene: C; Lpath: C; Merck: C; Regeneron: C; Second Sight Medical Products: C; ThromboGenics: C: KalVista: C.
See the disclosure key at www.aao.org/eyenet/disclosures.
|