Download PDF
Autoimmune retinopathy (AIR) comprises a spectrum of retinal degenerative disorders that includes the paraneoplastic (PAIR) and nonparaneoplastic (nPAIR) subtypes. Both subtypes are triggered by molecular mimicry, a phenomenon in which there are similarities in sequence between foreign antigens and self-antigens such that self-antigens can cause an immune response. However, in PAIR, this molecular mimicry occurs between tumor antigens and retinal proteins, whereas in nPAIR, the mimicry is between retinal proteins and presumed infectious (bacterial or viral) antigens. Both subtypes of AIR lead to retinal degeneration and may result in visual loss.1
The PAIR category is further subdivided into cancer-associated retinopathy (CAR) and melanoma-associated retinopathy (MAR); nPAIR, by definition, is not associated with underlying malignancy and is more likely to occur in patients with a history of autoimmune disease.2
AIR Overview
Pathophysiology. Antiretinal antibodies (ARAs) are believed to be intimately linked to the pathogenesis of AIR. Depending on the specific disease subtype, the antibodies target different retinal antigens.3 However, the isolated finding of ARAs is not pathognomonic for AIR, as they may occur as normal variants in the population or manifestations of other processes such as uveitis or even age-related cataracts.3 Moreover, some AIR cases have been reported in which no ARAs were found.2
Diagnosis. Diagnosing AIR is challenging because its signs and symptoms are nonspecific, overlapping with many other retinal conditions. The differential diagnosis includes hereditary retinal disease, various retinal degenerative disorders, toxic retinopathies, inflammatory diseases, infectious causes, and diffuse unilateral subacute neuroretinitis.2
The diagnosis of AIR is made based on a careful history and clinical examination; visual field testing; multimodal imaging, including optical coherence tomography (OCT), fundus autofluorescence, and fluorescein angiography; full-field electroretinography (ffERG); retinal antibody testing; and malignancy evaluation.4
Although techniques such as immunohistochemistry (IHC), enzyme-linked immunosorbent assay (ELISA), and Western blot are effective in determining the presence of autoantibodies, this information alone is not sufficient for the diagnosis of AIR4 and must be considered in conjunction with the history, workup, and clinical picture.2 In addition, all patients who present with autoimmune retinopathy without a history of cancer should be thoroughly examined to rule out an occult malignancy.2
Management. The different AIR subtypes have similar treatments, with immunosuppression as the mainstay of therapy. Follow-up testing, including assessment of visual acuity, color vision, visual field, OCT, and ffERG, is generally done at 3-month intervals. However, this interval may be adjusted depending on treatment type, patient symptoms, and course of the condition.5
(click to expand)
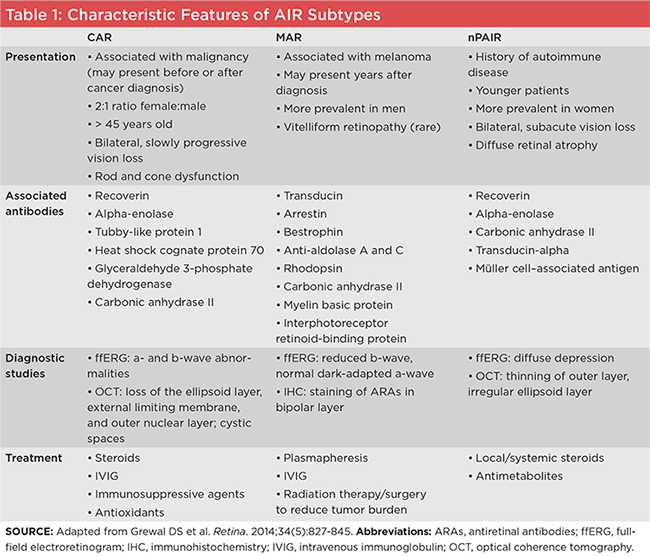
Cancer-Associated Retinopathy
CAR (Fig. 1) has been observed in conjunction with many different types of tumors, including small cell lung carcinoma (the most frequent association), cervical cancer, mixed Müllerian tumor, endometrial carcinoma, and uterine sarcoma.1 CAR is most commonly associated with the anti-recoverin antibody. Recoverin is a retina-specific calcium-binding protein in photoreceptors.3 Anti-recoverin antibodies induce an increase in intracellular calcium, leading to retinal apoptosis and, ultimately, retinopathy through caspase-dependent pathways.4 Other autoantibodies that have been associated with CAR include antibodies against alpha-enolase, tubby-like protein 1, heat shock cognate protein 70, glyceraldehyde 3-phosphate dehydrogenase, and carbonic anhydrase II.3
Patient presentation. CAR may present either after or before the diagnosis of cancer. CAR generally develops after the age of 45, and women are affected more than men at a 2:1 ratio.1 It presents with bilateral, slowly progressive vision loss. Patients will have signs and symptoms related to both rod and cone dysfunction, such as glare, decreased color perception, scotomas, light aversion, nyctalopia, and peripheral visual field deficits. Optic disc pallor may develop later in the disease.1
Diagnosis. CAR is a clinical diagnosis based upon the symptoms and findings described above, most often in the context of a clinically identified carcinoma. ffERG may show abnormalities in both a- and b-waves. On OCT, cystic spaces may be seen, as well as loss of the ellipsoid layer, external limiting membrane, and outer nuclear layer.1 Identification of anti-recoverin antibodies (the most prevalent autoantibody) or other ARAs by means of IHC, ELISA, or Western blot in the setting of carcinoma can point toward a diagnosis of CAR. The presence of ARAs and the visual symptoms can precede the diagnosis—and may prompt an investigation—of the systemic cancer. Because certain tumors typically express specific antibodies, information on the type of antibody present may be useful in diagnosing the underlying cancer.1
Treatment. Of the AIR subtypes, CAR typically is the most responsive to treatment. Therapy for CAR includes corticosteroids, other immunosuppressive agents, intravenous immunoglobulin (IVIG), and antioxidants.2 Treatment outcomes are variable and depend on the severity of damage to the photoreceptors. Triple therapy with an initial dosage of 100 mg/day cyclosporine, 100 mg/day azathioprine, and 20 to 40 mg/day prednisone has been reported to be associated with improvement in visual acuity or an increased visual field.1
 |
|
CANCER-ASSOCIATED RETINOPATHY. Note severe vascular attenuation without obvious pigmentary changes in the fundus of a patient with ovarian carcinoma.
|
Melanoma-Associated Retinopathy
Typically, MAR is observed in patients in whom melanoma has already been diagnosed and has metastasized. MAR is most commonly associated with melanomas of cutaneous origin or uveal melanoma.4 MAR is more common in men than in women.1 It has been associated with autoantibodies against bipolar cells, bestrophin, enolase, myelin basic protein, aldolase, rhodopsin, and arrestin, among others.4
Patient presentation. Patients with MAR may present with visual symptoms many years after the diagnosis of melanoma. They commonly experience photopsia and peripheral field loss; they may also complain of nyctalopia.4 Visual symptoms progress over time.
Fundus examination may be unremarkable or may reveal optic nerve pallor, retinal pigment epithelium abnormalities, and vessel changes. Some patients with melanoma have a rare type of retinopathy that presents with vitelliform lesions and serous retinal detachment.1
Diagnosis. As with CAR, diagnosis depends largely on the clinical picture. The indirect IHC staining of ARAs in the bipolar layer can be diagnostic. ERG abnormalities are present, with the characteristic ffERG pattern being a reduced b-wave and a normal dark-adapted a-wave due to the dysfunction of the “on” bipolar cells.3
Treatment. Most of the treatment modalities for MAR are not supported by evidence of definitive improvement.5 Plasmapheresis is intended to remove the antibodies and cytokines involved in the pathogenesis from the blood, but patients in a trial that investigated plasmapheresis also received other treatments; thus, it is difficult to determine its effect in isolation.5 IVIG may be used at a dose of 1 g/kg twice daily to shorten the half-life of the antibodies.5 Radiation therapy and surgery may also be useful to reduce tumor size and, thus, the load of antibodies.
nPAIR
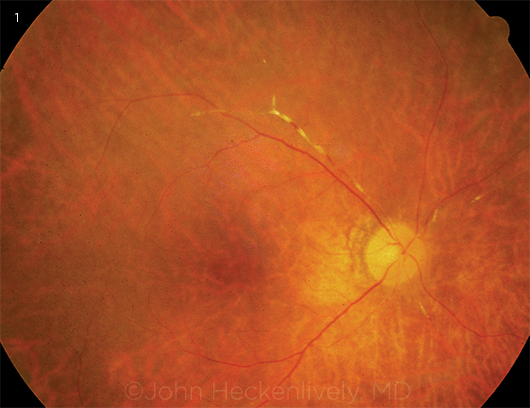
Nonparaneoplastic autoimmune retinopathy is the most common subtype of AIR. There are various diseases on the spectrum of nPAIR, such as acute zonal occult outer retinopathy (AZOOR), an inflammatory disorder (Fig. 2). Recoverin, Müller cell–associated antigen, alpha-enolase, CAII, inner retinal layer, and rod transducin are all ARAs that have been associated with nPAIR.3
Patient presentation. The clinical picture of nPAIR is similar to that of CAR, although nPAIR patients tend to be younger and have a family history of autoimmune disease.5 Typically, nPAIR presents bilaterally. Symptoms include subacute vision loss, photopsias, scotomas, and/or decreased color vision. Fundus examination may be unremarkable or may demonstrate optic disc pallor. Visual acuity is usually preserved early in the course of the disease process. Most patients have diffuse retinal atrophy.2
Diagnosis. To make the diagnosis of nPAIR, the clinician must rule out malignancy and retinitis pigmentosa (RP), as their clinical presentations are similar to nPAIR.1 The presence of ARAs is also critical; many patients may present with multiple antibodies, which aid in the diagnosis of nPAIR. Diffuse depression on ffERG and thinning of the outer nuclear layer with irregularity of the ellipsoid layer may be seen on OCT.2 Finally, an absence of retinal degeneration, fundus lesions, or intraocular inflammation further points toward the diagnosis of nPAIR.2
Treatment. Local or systemic steroids and antimetabolites are first-line treatments in the management of nPAIR. Monoclonal antibodies (such as rituximab) or IVIG can be considered as second-line therapies. However, therapy has not been shown to be helpful if retinal degeneration has already occurred.2
 |
|
SD-OCT IN AZOOR. (2A) Parafoveal outer retinal loss with central sparing (“flying saucer sign”). (2B; different patient) Diffuse loss of the retinal photoreceptor inner and outer segments.
|
Conclusion
The diagnosis of AIR can be difficult and requires a thorough workup. Although the presence of ARAs can be helpful in supporting or prompting the workup of malignancy, a positive test for ARAs is not independently diagnostic. Once the clinician has made the diagnosis, treatment primarily involves immunosuppression. Measurement of ARA titers has not been demonstrated to be useful in clinical practice; improvement is judged based on serial visual field and ERG assessments. Further research is warranted to guide treatment and to gain a better understanding of prognosis associated with each of the AIR subtypes.
___________________________
1 Grewal DS et al. Retina. 2014;34(5):827-845.
2 Grange L et al. Am J Ophthalmol. 2014;157(2):266-272.
3 ten Berge JC et al. PLoS One. 2016;11(12):e0167909. doi:10.1371/journal.pone.0167909.
4 Braithwaite T et al. Ophthalmologica. 2012;228(3):131-142.
5 Fox AR et al. Am J Ophthalmol. 2016;168:183-190.
___________________________
Ms. Khanna is a medical student at the University of Wisconsin School of Medicine and Public Health, in Madison. Dr. Ringeisen is a retina fellow at VitreoRetinal Surgery PA, in Minneapolis. Dr. Mititelu is an assistant professor, in the Department of Ophthalmology and Visual Sciences, University of Wisconsin School of Medicine and Public Health. Relevant financial disclosures: None.