NOTE: This article has been updated since print publication. Figure 3 shows a different image of Fleischer ring.
Download PDF
Keratoconus (KC) is a bilateral, progressive, noninflammatory ectatic condition in which there is conical protrusion of a thinned central cornea. Patients experience significant visual impairment from the resultant irregular astigmatism and high myopia. The worldwide prevalence of this condition is estimated to be 1.38 per 1,000.1 KC has been found to affect all ethnicities, although the prevalence and incidence are higher among South Asians and Middle Easterners compared with those of European ancestry.2 The condition affects both sexes, and there are contradictory studies on whether the prevalence differs significantly between the sexes.3
Etiology and Pathogenesis
KC is a complex disease with a multifactorial etiology, likely encompassing both genetic and environmental factors. Although only 8% to 10% of KC patients have a family history of the disease, a genetic basis for the condition is supported by autosomal dominant and recessive patterns of inheritance, association with other genetic disorders, and twin concordance studies.3 Numerous candidate genes have been identified through genomic studies.
Mechanical and other risk factors are also implicated in the development of KC. These include eye rubbing, trauma from poorly fitting contact lenses, and allergic eye disease.3
The pathophysiology of KC is not completely understood. Biochemical instability leading to central or paracentral stromal thinning has been attributed to an imbalance between proteolytic enzymes and proteinase inhibitors.3
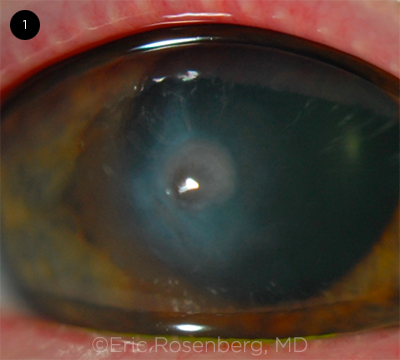 |
|
ACUTE COMPLICATION. A case of acute corneal hydrops, with the cornea demonstrating marked localized edema.
|
Presentation and Course
Symptoms. Although KC is bilateral, it typically progresses asymmetrically. Patients commonly present with complaints of blurring, distorted vision, and frequent change in spectacle prescriptions. Other symptoms include glare, photophobia, and distorted night vision. In advanced KC, high myopia, irregular astigmatism, and stromal scars lead to significant visual impairment.
Onset and progression. The onset of KC typically occurs around the second decade of life, with the disease progressing slowly thereafter and ceasing in most patients by the fourth decade. Early in the disease, KC is asymptomatic, and many cases remain undiagnosed unless assessed by corneal tomography. Although several indices are available to monitor the progression of keratoconus, there is no consensus on which is most reliable.3
Complications. Acute corneal hydrops, the development of stromal edema following a break in the Descemet membrane, is a potential complication of KC (Fig. 1). It presents with a rapid onset of pain and loss of vision. Although corneal hydrops may resolve spontaneously within six to 10 weeks, many patients ultimately require keratoplasty because of corneal scarring.3
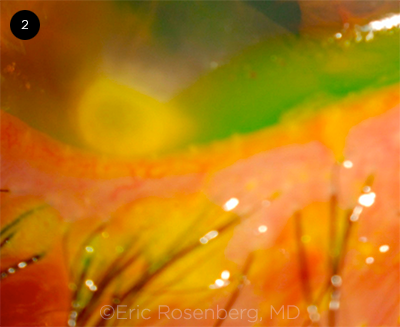 |
|
EYELID SIGN. On infraduction of the globe, the lower lid of this keratoconic patient exhibits a characteristic V-shaped Munson sign.
|
Diagnosis
Several important clinical features can aid in the diagnosis of KC.
Examination. Scissoring of the red reflex on retinoscopy is a reliable and sensitive method for detecting early-stage KC.
External indicators include the Munson sign (V-shaped deformation of the lower eyelid caused by the cone when the patient looks down; Fig. 2) and Rizzuti sign (conical illumination on the nasal sclera when light is directed on the cornea from the temporal side). However, these external signs are typically not observed in mild KC.
Slit-lamp evaluation. Examining the patient at the slit lamp may reveal several key diagnostic features of KC. Central and paracentral thinning of the cornea is a characteristic sign. The Fleischer ring, a yellow or brown ring encircling the cone, is caused by the deposition of hemosiderin; it is best appreciated with a cobalt blue light filter (Fig. 3). Vogt striae, which are often seen in the deep stroma, are bright, parallel stress lines caused by the tension of corneal stretching. External pressure on the globe eliminates these lines on slit-lamp examination. In addition, corneal nerves can be visualized as fine white lines entering into the stroma from the limbus.
Topography and tomography. Corneal topography and tomography provide valuable information about the corneal curvature. Corneal topography allows noninvasive qualitative and quantitative characterization of corneal morphology. Topographic maps will show irregular astigmatism with steepening. The following maps are analyzed: anterior, sagittal, and tangential curvature maps; anterior and posterior elevation maps; and the thickness map.4
Corneal tomography provides additional parameters for evaluating the anterior and posterior corneal surfaces. Early posterior corneal structural changes, including stromal thinning and elevation changes, are observed prior to anterior surface changes in KC.4 This allows for reliable detection of early-stage KC even before a patient becomes symptomatic.
Other techniques. Other adjunctive technologies can aid in confirming the diagnosis of KC. One of these, the Ocular Response Analyzer (Reichert), evaluates corneal biomechanics by measuring corneal hysteresis, the difference in applanation pressure when the cornea bends inward in response to a jet of air and when it returns to its normal state.4 Compared with normal corneas, keratoconic corneas typically exhibit lower corneal hysteresis values.4
High-resolution optical coherence tomography (OCT) is a useful and rapid diagnostic adjunct modality that allows analysis and mapping of the thickness of the corneal epithelium. Epithelial mapping has shown increased overall peripheral epithelial thickness with thinner central epithelium in keratoconic eyes compared with normal eyes.5 These changes may occur early in the disease process and are thought to be a compensatory mechanism.5
Associated Disorders
KC may be associated with systemic and ocular conditions. Patients with any of the disorders listed below should be carefully assessed for early signs of KC.2
Systemic associations include
- Down syndrome
- Ehlers-Danlos syndrome
- Leber congenital amaurosis
- Marfan syndrome
- Mitral valve prolapse
- Obstructive sleep apnea
- Osteogenesis imperfecta
- Turner syndrome
Ocular associations include
- Aniridia
- Blue sclerae
- Retinitis pigmentosa
- Vernal keratoconjunctivitis
|
Classification
Morphologically, KC is differentiated into three types of cones increasing in size: 1) small, isolated, round cones with steep curvature; 2) ellipsoid oval cones; and 3) large globus cones that cover the majority of the cornea.
Grading. The oldest and most commonly used grading system, the Amsler-Krumeich scale, is based on corneal thickness, anterior keratometric measurements, and refractive error.
The more recently developed classification known as the ABCD grading system incorporates average anterior radius of curvature (A) and posterior average radius of curvature (B), both measured in a 3-mm zone centered on the thinnest point of the cornea, along with thinnest pachymetry measurement (C), and best spectacle-corrected distance visual acuity (D).6 This system integrates tomographic values and visual acuity to better characterize the anatomic and functional aspects of keratoconic corneas.6
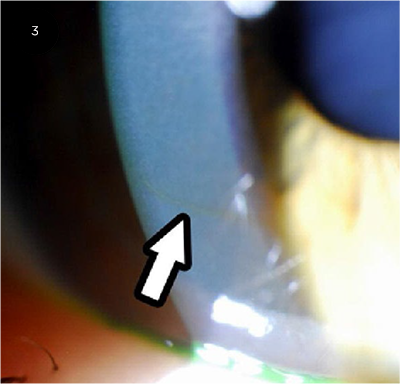 |
|
SLIT-LAMP SIGN. Slit-lamp photograph reveals a corneal Fleischer ring composed of iron deposits in a patient with KC.
|
Differential Diagnosis
Several corneal ectatic disorders require careful differentiation. Forme fruste keratoconus (subclinical KC) is an early, asymptomatic form of the disease with no apparent clinical signs; it can be diagnosed only through analysis of corneal morphology.3
Pellucid marginal degeneration (PMD) is a bilateral, noninflammatory ectatic disorder similar to KC. Clinically, PMD patients are typically asymptomatic, except for slow, progressive reduction in visual acuity refractory to spectacle correction. PMD is characterized by inferior corneal thinning, typically in a band-line area concentric to the limbus on slit-lamp evaluation and a “crab-claw” appearance on topography.7
Keratoglobus is a corneal thinning disorder characterized by global thinning and protrusion. Unlike KC, it is typically nonprogressive and present from birth. While the thinning in KC is focal, keratoglobus demonstrates protrusion and thinning of the entire cornea and is more prominent in the periphery than is KC.7
Management
A number of approaches have been developed to improve the quality of vision in affected patients and, in some cases, to slow or stop disease progression. The choice of therapy depends on the severity of the disease and the age of the patient, as well as the contraindications and possible complications of these treatment modalities. Keratoconic patients in their third decade of life should be followed every six months. Patients with higher risk factors, including pregnancy or young age (under 20 years), require evaluation every three months.8 Patients with severe KC often require combination therapy.
Spectacles and contact lenses. Spectacles can be used to correct astigmatism in early-stage, stable KC. When the astigmatism can no longer be managed with glasses, contact lenses are the next step. Soft contact lenses may be sufficient in mild KC, with rigid gas-permeable contact lenses becoming necessary in more advanced disease. However, although many designs are available, conventional contact lenses may be uncomfortable on a keratoconic eye, and patients may experience dryness, itching, and pain.8
Scleral lenses. Unlike conventional contact lenses that rest directly on the cornea, scleral lenses have a larger diameter and rest on the sclera, vaulting over the cornea. With these lenses, there is a fluid layer between the lens and cornea. The PROSE (prosthetic replacement of the ocular surface ecosystem; BostonSight) treatment incorporates a scleral lens customized for each patient. Although scleral lenses have a higher cost and more challenging fitting process, they offer increased stability, improved visual outcomes, and better comfort compared with standard contact lenses.9
Collagen cross-linking. The technique of collagen cross-linking (CXL) with ultraviolet A and riboflavin stabilizes corneal tissue, halting or arresting disease progression.10 In addition, CXL has been found to improve BCVA by 1 to 2 lines and reduce maximum keratometry (Kmax) by 1 to 2 D.10 Currently, CXL is recommended for patients with progressive KC who have a clear cornea and a minimum corneal thickness of 400 μm. The advent of this modality has reduced the need for keratoplasties.11 Adverse effects include infectious keratitis, edema, and haze.10
In early U.S. studies, custom topography-guided photorefractive keratectomy has been used as an adjunct to improve visual function and normalize remaining corneal surface abnormalities. This treatment should be deferred until the cornea has stabilized, at least three months after CXL.12
Intracorneal ring segments. Intracorneal ring segments (ICRS) made of polymethyl methacrylate can be implanted into deep corneal stroma. Through an arc-shortening effect, ICRS flatten the corneal surface, reducing the refractive error.13 The amount of refractive correction depends on the diameter and thickness of the rings. Shorter and thinner arcs are used to correct astigmatism, while longer and thicker arcs correct myopia. Complications of ICRS include fluctuating visual outcomes, infection, dysphotopsias, and corneal melting.13
Keratoplasty. When less-invasive procedures are not effective, patients may require corneal transplantation. Penetrating keratoplasty (PK) for KC is an effective procedure with good visual outcomes. Recovery takes several weeks to months, with visual function stabilizing up to one year after surgery. Reported complications include allograft rejection, iatrogenic astigmatism, and recurrence of KC. Up to half of transplanted eyes will require contact lens correction for full visual rehabilitation.14
To preserve unaffected native endothelial cells, surgeons may perform a deep anterior lamellar keratoplasty (DALK) if the Descemet membrane has not been previously ruptured, as in hydrops. While the visual outcomes are comparable to PK, DALK eliminates the risk of endothelial rejection and steroid-induced secondary glaucoma. However, Descemet membrane perforation is a potential intraoperative complication that may require conversion to PK, and interface haze may limit full visual recovery.14
Conclusion
Over the past two decades, technological advancements have improved the early diagnosis and management of KC. The diagnostic workup should involve a detailed medical history, a thorough slit-lamp examination, and imaging analysis techniques such as tomography and OCT. Treatment plans remain patient specific and should be based on a collaborative discussion that appropriately addresses the individual’s concerns and expectations for visual outcome.
__________________________
1 Hashemi H et al. Cornea. 2020;39(2):263-270.
2 Bialasiewicz A, Edward DP. Middle East Afr J Ophthalmol. 2013;20(1):3-4.
3 Mas Tur V et al. Surv Ophthalmol. 2017;62(6):770-783.
4 Martinez-Abad A, Piñero DP. J Cataract Refract Surg. 2017;43(9):1213-1227.
5 Li Y et al. J Cataract Refract Surg. 2016;42(2):284-295.
6 Belin MW, Duncan JK. Klin Monbl Augenheilkd. 2016;233(6):701-707.
7 Martinez-Abad A, Piñero DP. Cont Lens Anterior Eye. 2019;42(4):341-349.
8 Gomes JA et al. Cornea. 2015;34(4):359-369.
9 Koppen C et al. Am J Ophthalmol. 2018;185:43-47.
10 Raiskup F et al. J Cataract Refract Surg. 2015;41(1):41-46.
11 Sandvik GF et al. Cornea. 2015;34(9):991-995.
12 Nattis A et al. J Cataract Refract Surg. 2018;44(8):1003-1011.
13 Park SE et al. Curr Opin Ophthalmol. 2019;30(4):220-228.
14 Mohammadpour M et al. J Curr Ophthalmol. 2017;30(2):110-124.
Mr. Nuzbrokh is a fourth-year medical student and Dr. Rosenberg is a cornea and refractive surgery fellow; both are in the Department of Ophthalmology at Weill Cornell Medical College, New York, N.Y. Dr. Nattis is a cornea specialist and director of research at SightMD, Babylon, N.Y. Relevant financial disclosures: Mr. Nuzbrokh and Dr. Rosenberg: None. Dr. Nattis: Alcon: C,L,S.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Yan Nuzbrokh None.
Dr. Rosenberg None.
Dr. Nattis Alcon: C,L,S; Glaukos: S.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|