By Mahera Husain, BS, Steve Gerber, MD, and Amir Hajrasouliha, MD.
Edited By: Gregg T. Kokame, MD, and Bennie H. Jeng, MD.
NOTE: This article has been updated since print publication. In the original article, EyeNet incorrectly stated that Fig. 3A shows a detached posterior vitreous cortex. In fact, the patient had an attached posterior vitreous during the surgery. This version of the article corrects the mistake.
Download PDF
Macular holes (MH) result in central vision loss, metamorphopsia, and a central scotoma. Idiopathic macular holes (IMHs) account for 83% of cases; they are found more commonly in females and are associated with increased age. Trauma is another cause, with traumatic macular holes (TMHs) comprising 5% to 8% of total cases.1 It is important to consider TMH as a cause of vision loss following trauma, as it will inform medical and surgical management.
 |
|
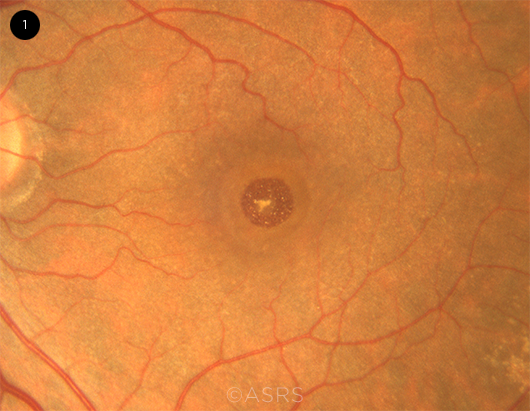
MACULAR HOLE. Fundus photograph shows a full-thickness MH in an elderly woman.This image was originally published in the ASRS Retina Image Bank. Gary R. Cook, MD, FACS. Full-Thickness Macular Hole. Retina Image Bank. 2019; Image Number 29967. © The American Society of Retina Specialists.
|
Pathophysiology
Idiopathic. IMHs are most common in women over the age of 65, and they are due to tangential traction by the cortical vitreous on the fovea. The retina is thinnest at the foveal floor, and progressive traction results in elevation of the fovea—and, subsequently, an opening in the foveal tissue, which then results in a full-thickness MH (Fig. 1).
Traumatic. A TMH has a different etiology from IMH. TMH occurs when an acute blunt force on the globe results in a contrecoup injury on the macula. MH develops due to a contusion on the retina with secondary necrosis, subfoveal hemorrhage, and acute vitreous traction resulting from the acute flattening of the posterior globe. Trauma can occur with sports injuries, work-related accidents, lightning strikes, and electrical shock. With the 4th of July celebrations, fireworks-related injuries are also a common cause of TMH.
 |
|
LAMELLAR HOLE. OCT shows a partial-thickness hole and a large subfoveal druse. The patient had 20/25 visual acuity.
|
Diagnosis and Classification
MH is diagnosed by means of a thorough history and clinical examination including slit-lamp biomicroscopy of the fundus with a dilated pupil. Symptoms usually include blurred central vision, inability to read or watch television, and distortion of the central vision with metamorphopsia. Visual acuity is decreased, and Amsler grid testing shows the central scotoma and metamorphopsia. At the slit lamp, a Watzke-Allen sign shows a break noted by the patient when a thin slit-lamp beam is focused across the MH. OCT provides the detailed imaging to make the diagnosis of full-thickness MH, which shows a break involving all the different layers of the retina.
Differential diagnosis. OCT can be used to differentiate between true MH and conditions that may appear similar on clinical examination, including lamellar macular hole and pseudohole, among others.2
Lamellar macular holes have an irregular contour with a break in the inner fovea without a break in the outer retina. There are often overhanging edges over the inner foveal defect on OCT. The Watzke-Allen sign is negative.
Macular pseudoholes associated with epiretinal membranes appear as a central reddish spot in the fovea with a surrounding epiretinal membrane. On OCT, the outer retina is intact, and the inner retinal defect often has steep edges. The Watze-Allen sign is negative.
Vitreomacular traction syndrome can result in tractional macular cystic changes but often is associated with many areas of traction on the retina outside the fovea.
Other less common causes of diagnoses simulating a macular hole, especially in its early stages, include subfoveal drusen, central serous chorioretinopathy, cystoid macular edema, and vitelliform lesions resulting in a central yellow spot typical of stage I macular holes (see “Classification,” below) with traction on the fovea resulting in increased visualization of the xanthophyl pigment.
Classification. There are two main systems for classifying MHs.
The Gass system (1988; updated in 1995) divides MHs into various stages based on fundus biomicroscopy. The IMH progresses through stages as initially described by Gass in his biomicroscopic examinations.3
- Stage I is a foveal elevation without a break.
- Stage II is foveal elevation with a break either in the central fovea or at the peripheral edge of the elevated fovea.
- Stage III is a full-thickness macular hole.
- Stage IV is a full-thickness macular hole with complete posterior vitreous detachment.
The International Vitreomacular Traction Study (IVTS) classification (2013) is based on OCT appearance. It considers size (horizontal diameter at narrowest point) and status of the vitreomacular interface. Full-thickness MHs are categorized by size as small (≤250 μm), medium (>250 μm to ≤400 μm), or large (>400 μm). They are further characterized by vitreous status (with or without vitreomacular traction) and by cause (primary or secondary).4 Full-thickness macular hole is primary if caused by vitreous traction, and secondary if the macular hole is caused by pathologic characteristics other than vitreomacular traction.
Case Study: Firecracker Trauma
A 12-year-old boy was playing with firecrackers when he threw one down and a rock shot up, striking him in the eye, causing immediate pain and blurred vision. When seen in the office the next day, he had a VA of counting fingers and extreme photophobia in that eye. He also had 2+ anterior chamber cells, a superficial central corneal abrasion, and no hyphema. He was put on topical prednisolone acetate every 2 hours.
He was feeling much better when he returned the next day, but his vision had not improved, despite a clear cornea and much-reduced inflammation. An OCT was ordered because of the unexplained vision loss. The OCT showed a MH (>1,500 μm) with attached posterior vitreous, which was confirmed intraoperatively (Fig. 3A).
Surgery. The decision was made to perform surgery because of the large size of the hole. A 25-gauge pars plana vitrectomy was performed. The ILM was stained with brilliant blue dye, and ILM peel was performed from arcade to arcade with a retractable Tano scraper and ILM forceps. A retinal break was noted at the 7-o’clock position, as well as lattice degeneration. Endolaser was used to seal both the break and the lattice degeneration. The final step was air-fluid exchange with 15% perfluoropropane (C3F8).
Follow-up. At the six-week postoperative follow-up, the patient still had 15% C3F8 gas in the eye with the closed MH, and vision had improved to 20/100+2. At the six-month postoperative appointment, the patient’s VA was 20/200. Although the hole was anatomically closed, vision was decreased as a result of photoreceptor loss (Fig. 3B).
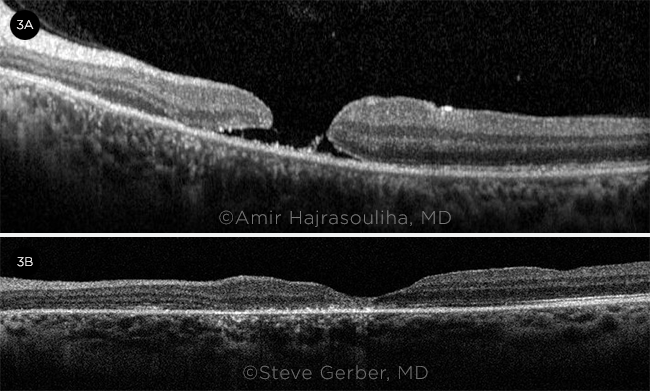
TRAUMA AND TREATMENT. (3A) Macular OCT taken two days after injury shows a MH (>1,500 μm). (3B) Image taken six months postoperatively shows repaired MH with overall thinning centrally due to photoreceptor loss.
|
Management
Management of MH depends on the etiology, the size of the hole, and, in the case of trauma, the presence of associated ocular complications such as vitreous hemorrhage, choroidal ruptures, and commotio retinae.
Observation. Small idiopathic holes (≤250 μm) may close spontaneously, and this may be facilitated by the use of topical medications to decrease macular edema. TMHs appear to have a higher rate of spontaneous closure than do IMHs. Because of the higher chance of spontaneous closure of TMHs, which can occur most commonly over two to six months, initial management is often observation.5 However, if TMHs persist, these holes can be managed by the same type of surgery utilized for IMHs.6
Surgery. Surgical technique for MHs has evolved since the initial description by Kelly and Wendel in 1991.7 Vitrectomy, membrane peeling, and the use of an intraocular tamponade, such as SF6 gas, C3F8 gas, or silicone oil, are utilized to relieve traction on the MH and to stimulate closure of the MH. Peeling of the internal limiting membrane (ILM) is performed by many vitreoretinal surgeons. This step often utilizes agents to assist in peeling including brilliant blue dye, indocyanine green dye, and triamcinolone.
Recently for larger or recurrent MHs, different substrates have been put into the MH to facilitate closure by providing a scaffold for glial proliferation. An inverted flap technique involves peeling of the ILM and inverting the ILM flap into the MH or over the MH.8 One randomized trial compared the inverted flap technique to conventional peeling in holes larger than 600 μm and found significantly higher rates of anatomic success (90% vs. 70%, respectively) and visual recovery (2.1 lines vs. 1.4 lines, respectively) with the inverted flap method.9 ILM transplantation from other parts of the macular region may also facilitate closure.10 In very large MHs, retinal transplantation into the hole is used by an autologous retinal graft from the retinal periphery.11
Postoperative positioning has traditionally been face down, which floats the bubble directly to the macular region. However, many surgeons feel comfortable not putting their patients face down and feel that the gas bubble contact even in the sitting-up position allows for adequate tamponade.
Fireworks Trauma Prevention
- Fireworks are dangerous explosives and often result in trauma not only to the person lighting the fireworks but also to bystanders. It is important to be aware of your surroundings and where fireworks are being lit.
- Use protective eyewear.
- Keep unused fireworks in a safe place away from children.
- Never return to a lit firework and, especially, do not look down over a lit firework. Do not relight a dud.
|
Outcomes
Visual outcomes are best for patients who were symptomatic for less than six months before surgery. MHs that are larger than 1,200 μm have a greater chance of failing to close after surgery. Chronic MHs, which have persisted for longer than two to three years, have a poorer visual prognosis even when the MH closes. However, closure of MHs in the majority of cases has resulted in improved visual acuity and decrease in metamorphopsia. There is a small chance of MHs reopening, but vision improvement remains long-term in most patients.
Conclusion
Surgery for MHs has developed over the past 30 years into a very successful procedure to improve vision by closure of the MHs. Symptoms of MHs include blurred vision, a central scotoma, and metamorphopsia. Fundus examination shows a defect in the fovea and is confirmed with OCT showing a defect in all layers of the retina. Surgical techniques have developed to increase closure rates in larger MHs. TMHs have a different etiology than IMHs and have a higher chance of spontaneous closure, so observation is often recommended. However, the most important recommendation is to prevent ocular trauma, especially with fireworks that may be used to celebrate the 4th of July.
__________________________
1 Gao M et al. Curr Eye Res. 2017;42(2):287-296.
2 Chen JC, Lee LR. Br J Ophthalmol. 2008;92(10):1342-1346.
3 Gass JDM. Am J Ophthalmol. 1995;119:752-759.
4 Duker JS et al. Ophthalmology. 2013;120:2611-2619.
5 Kusaka S et al. Am J Ophthalmol. 1997;123:937-839.
6 Johnson RN et al. Ophthalmology. 2001;108:853-857.
7 Kelly NE, Wendel RT. Arch Ophthalmol. 1991;109:654-659.
8 Michalewska Z et al. Ophthalmology. 2010;117:2018-2025.
9 Baumann C et al. Retina. 2020;40:1955-1963.
10 Morizane Y et at. Am J Ophthalmol. 2014;861-869.
11 Grewal DS et al. Ophthalmology. 2019;125:1399-1408.
__________________________
Ms. Husain is a third-year medical student at the Indiana University School of Medicine in South Bend. Dr. Gerber is in practice at Advanced Ophthalmology in Michiana, Ind., and is a clinical professor of ophthalmology at Indiana University School of Medicine in South Bend. Dr. Hajrasouliha is a vitreoretinal specialist and assistant professor of ophthalmology at Indiana University School of Medicine in Indianapolis. Financial disclosures: None.