By Nasreen Raees Ahmed, MBBS, Radhika Tandon, MD, FRCSEdin, and M. Vanathi, MD
Edited by Sharon Fekrat, MD, and Ingrid U. Scott, MD, MPH
Download PDF
Aniridia is a panocular disorder characterized by varying degrees of iris hypoplasia, reduced visual acuity, and nystagmus secondary to foveal hypoplasia. Other ocular findings include cataract, glaucoma, aniridic keratopathy, and optic disc hypoplasia. It may occur as an isolated finding or may be associated with systemic involvement. The prevalence of aniridia has been reported to be 1 in 40,000 to 100,000, with no gender predilection.
Genetics and Inheritance
Two-thirds of aniridia cases are dominantly inherited with high penetrance, while the remaining one-third are sporadic with de novo mutations. In 90 percent of cases, there is loss of function of one copy (haploinsufficiency) of the PAX6 gene located on chromosome 11p13. The loss of function is attributed to intragenic mutations in two-thirds of cases and chromosomal rearrangements in the remainder. The PAX6 gene is hypothesized to be the master control gene for morphogenesis of the eye; it is also involved in the development of the brain, olfactory bulb, gut, and pancreas. The gene encodes for a transcriptional regulator with two DNA-binding domains, one of which is a paired domain, while the other is a homeodomain.
Intragenic mutations most commonly include insertion of a premature termination codon, leading to null mutations. C terminal extensions are also known to occur, which cause the open reading frame to continue into the 3′ untranslated region. Both of these mutations lead to severe forms of aniridia. Missense mutations due to single amino acid substitutions lead to less severe or variant forms of aniridia.
Chromosomal abnormalities such as deletions involving the PAX6 region, inversions, and translocations involving the transcription unit can also lead to aniridia. Deletion of the contiguous WT1 gene on chromosome 11p13 leads to the first of the two hits required to inactivate both alleles of WT1, leading to WAGR syndrome.
Other genes implicated in aniridia include FOXC1, PITX2, and PITX3.
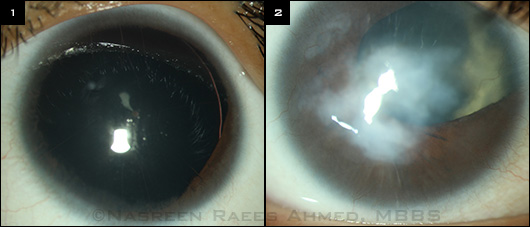 |
|
ANIRIDIA AND A VARIANT. (1) Typical form of aniridia, marked by almost complete lack of iris tissue. (2) Atypical iris coloboma accompanied by keratopathy.
|
Clinical Features and Management
Visual Acuity
Although visual acuity is generally low, it is unrelated to the extent of iris hypoplasia. Myopia is seen in 64 percent of aniridia patients, and amblyopia in 37 percent.1
Management. Refractive correction significantly improves visual acuity in these patients. Amblyopia, if present, requires prompt treatment.
Iris
Iris hypoplasia of varying degrees may be present. The iris may be absent, with only a stump visible on gonioscopy; or there may be atypical iris coloboma (location other than inferonasal), iris hole, lack of iris crypts, or stromal hypoplasia that is evident only on retroillumination. Careful slit-lamp examination is necessary to identify the more subtle iris defects.
Management. Light sensitivity can be managed with tinted or photochromic glasses, and wide-brimmed hats may be helpful. (Also see the “Lens” section below for iris implants.)
Cornea
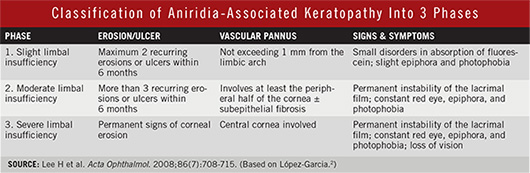
Aniridia-associated keratopathy (AAK) secondary to limbal stem cell deficiency (LSCD) occurs in 90 percent of patients. Features include increased central corneal thickness, superficial vascularization, subepithelial fibrosis, progressive corneal opacification, conjunctivalization, and a highly unstable ocular surface predisposing to recurrent epithelial erosions as well as bacterial ulcers and chronic inflammation. (See the table below for a classification of AAK.2)
Impression cytology may show goblet cells suggestive of conjunctivalization. The use of monoclonal antibodies to cytokeratins (CK) allows detection of specific cell lineages. CK 3 and 12, which are specific for corneal and limbal epithelial cells, are reduced in LSCD; while CK 19, which is specific for conjunctival epithelial cells, is increased. Confocal scanning reveals loss of basal and wing cell layers of the epithelium and reduction in subbasal nerves.3
Management. Patients with mild keratopathy can be managed with topical preservative-free lubricants. Moderate keratopathy is treated with autologous serum and amniotic membrane transplant.
In patients with severe phase 3 keratopathy, stabilizing the corneal surface involves replenishing limbal stem cells through transplantation. Since both eyes are affected, autografts cannot be used. Penetrating or lamellar transplants alone may not help, as the primary pathology is LSCD. Keratolimbal allografts or cultured limbal stem cells over amniotic membranes have been used. The Boston Keratoprosthesis has also been used successfully in AAK.
Lens
From 50 to 85 percent of patients develop cataracts by early adulthood. Subluxation of the lens due to weak zonules is more common in aniridic patients than in unaffected individuals. In addition, the anterior capsule appears to be more fragile.
Management. Cataract extraction improves vision, but the surgeon should be prepared to manage complications in eyes with weak zonular fibers.
Postoperatively, there may be rapid progression of keratopathy, secondary glaucoma, and macular edema.
Specialized intraocular lenses that incorporate an artificial iris diaphragm can be used to correct aniridia at the time of surgery. The approval status of these devices varies by country; for example, although they have been available in Europe for years, in the United States they can be implanted only with a compassionate use device exemption.
Intraocular Pressure and Glaucoma
Elevated intraocular pressure (IOP) may be seen in 50 to 75 percent of patients. It usually develops in late childhood and early adulthood as progressive anatomic changes occur in the angle. The angle appears to be open in infancy, with minimal to no coverage of the trabecular meshwork with remnant iris tissue. However, over time, the rudimentary iris stump rotates and covers the trabecular meshwork, resulting in raised IOP. The clinician should keep in mind that, due to increased central corneal thickness, IOP readings may be unreliable in patients with aniridia.
Routine gonioscopy should be performed regularly to monitor the encroachment of iris tissue on the trabecular meshwork. Visual fields should be assessed periodically.
Management. Initially, topical antiglaucoma medications may be used, but glaucoma associated with aniridia requires surgery in the majority of cases. Prophylactic goniotomy is quite effective in preventing or delaying the onset of glaucoma in young patients.
Trabeculectomy is generally the first surgery performed in aniridic glaucoma that is not controlled on medical treatment; however, glaucoma drainage devices are a reasonable alternative.4 Cyclodestructive procedures are reserved for refractory cases.
Retina and Optic Nerve
Poor vision and nystagmus in patients with aniridia are usually caused by foveal hypoplasia, which may be detected clinically as lack of a foveal reflex on direct ophthalmoscopy, reduced pigmentation, and crossing of vessels through the foveal avascular zone. Optical coherence tomography reveals an absence of foveal depression. In addition, 10 percent of aniridic patients have optic nerve hypoplasia.
Management. Although these conditions are not currently amenable to treatment, low vision rehabilitation services may be helpful. (See the “Additional Resources” box in this month's Practice Perfect.)
(click to expand)
Systemic Associations
WAGR syndrome. Sporadic aniridia is the most consistent finding in WAGR syndrome, which also includes Wilms tumor (pediatric nephroblastoma), genitourinary abnormalities, and mental retardation. In patients with WAGR, Wilms tumor tends to be bilateral and presents earlier than in those without the syndrome. Systemic evaluation should be performed in all children with aniridia, irrespective of family history.
Gillespie syndrome. In this rare autosomal recessive syndrome, aniridia is associated with ptosis, cerebellar ataxia, and mental retardation.
Other associations. Aniridia may also be associated with central auditory processing deficits causing hearing difficulties.
Genetic Counseling Considerations
Isolated aniridia. Two-thirds of patients with isolated aniridia have a positive family history. Because there is considerable phenotypic variability, the clinician must perform a careful slit-lamp examination on other family members before labeling the mutation de novo. If a parent is affected, each child has a 50 percent chance of having aniridia. If the parents of the proband are unaffected, risk to the siblings is low.
WAGR syndrome. Patients with WAGR syndrome are generally infertile; thus, reproductive transmission is significantly reduced. Large WAGR deletions usually arise de novo, so siblings are unlikely to be affected. Rarely, parents with balanced translocations may develop deletions and may transmit the deletion to 50 percent of their offspring.
___________________________
1 Valenzuela A, Cline RA. Can J Ophthalmol. 2004;39(6):632-638.
2 López-Garcia JS et al. Arch Soc Esp Oftalmol. 2006;81(8):435-444. [Article in Spanish.]
3 Le Q et al. Eye (Lond). 2013;27(6):763-766.
4 Allingham RR et al. Shields Textbook of Glaucoma. 6th ed. Philadelphia: Wolters Kluwer; 2010.
___________________________
Dr. Ahmed is a resident in ophthalmology; Prof. Tandon is Head of Unit, cornea, keratoplasty, and refractive services; Dr. Vanathi is associate professor, cornea, keratoplasty, and refractive services; all are at Dr. R.P. Centre for Ophthalmic Sciences, AIIMS, New Delhi, India. The authors report no related financial interests.