By Matthew E. Raecker, MD, Dong-Wouk Park, MD, and Andreas K. Lauer, MD
Edited by Sharon Fekrat, MD, and Ingrid U. Scott, MD, MPH
Download PDF
Degenerative myopia—also known as pathologic myopia—is defined as a refractive error of greater than –6.00 D with an axial length of more than 26 mm. Although myopia is a common disorder, with a prevalence of between 11 and 36 percent in developed countries, degenerative myopia is relatively uncommon. The condition accounts for 27 to 33 percent of the myopic population and corresponds to rates of 1.7 to 2.1 percent in the overall population.1 It is reported to be the seventh leading cause of blindness in the United States and Europe1 and the leading cause of blindness in Japan.2
Because as many as 10 percent of eyes with degenerative myopia may develop choroidal neovascularization (CNV), it is important for ophthalmologists to recognize this condition and to understand that management of myopic CNV differs from that of CNV due to age-related macular degeneration (AMD). This will be particularly true as globalization continues, even though regional variations in prevalence rates for pathologic myopia are likely to continue.
Clinical Features
The characteristic clinical appearance of the posterior pole in the myopic fundus is caused by stretching of the ocular layers as axial length increases. Classically, attenuation and absence of the retinal pigment epithelium (RPE) correspond to a peripapillary crescent with readily visible choroidal vessels and sclera.
Variable findings. Fundus manifestations vary by patient; for instance, the disc displays tilting and temporal flattening in 37.7 percent of these eyes. Pathologic studies indicate that a posterior staphyloma is present in as many as 35 percent of eyes, although this is frequently underestimated during clinical examinations.3 Histopathologic studies demonstrate that retinal degeneration is present in 11.4 percent of highly myopic eyes; over time, areas of atrophy can coalesce and result in geographic atrophy (GA).
Lacquer cracks representing ruptures in Bruch’s membrane occur in 4.2 percent of eyes with an axial length of greater than 26.5 mm and are most commonly found within staphylomatous areas of the posterior pole (Figs. 1A, 1B). Macular or foveal retinoschisis may also be present in highly myopic eyes, particularly in staphylomatous areas.1
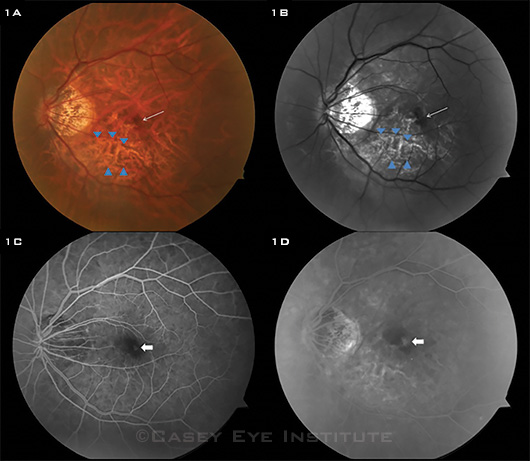 |
|
CLINICAL CLUES. This set of images is from a 55-year-old Asian woman with pathologic myopia. (1A, 1B) Color fundus and red-free photos reveal lacquer cracks (blue arrowheads). The optic disc displays tilting and temporal flattening, there is a posterior staphyloma, and macular hemorrhage (thin white arrow) is present near the lacquer cracks. (1C) Early fluorescein angiography image shows choroidal fluorescence blocked by hemorrhage with faint hyperfluorescence (white arrow). (1D) In the later angiographic image, increased central hyperfluorescence with leakage is observed, consistent with CNV. Also note overall greater transmission of scleral staining due to thinning of retina and choroid.
|
CNV and Risk of Progression
CNV. Choroidal neovascularization is frequently associated with lacquer cracks or areas of RPE atrophy. CNV has been reported to affect up to 10 to 15 percent of eyes with an axial length greater than 26.5 mm and is a major cause of vision loss among patients with myopic degeneration.
Although patients may be asymptomatic if CNV is located outside the central macula, symptoms of new-onset metamorphopsia, scotomata, and/or decreased vision are typical when CNV occurs centrally. Often, CNV is self-limited and becomes confined by migration of RPE cells. The resultant hyperpigmented spot, visualized on examination, has been termed Fuchs’ spot.1
The clinical course of CNV associated with degenerative myopia is different from that of CNV associated with AMD. This difference is important for the clinician to keep in mind when evaluating, treating, and counseling patients with myopic macular degeneration.
Progression. The natural progression of degenerative myopia has been documented in several studies. For instance, in a study that followed 806 eyes with axial lengths greater than 26.5 mm or refractive error greater than –8.00 D for an average of 12.7 years, the researchers observed progression of myopic fundus changes in 40.6 percent of eyes.
Moreover, 82 of 91 eyes (90.1 percent) that presented with CNV demonstrated progressive macular atrophy during an average follow-up of 11.8 years.2
In another study, 27 eyes with myopic CNV were followed for at least 10 years. At the initial visit, 19 of the eyes had vision better than 20/200. This decreased significantly, with 24 of the eyes having vision worse than 20/200 at the five-year mark, and 26 of the eyes having vision worse than 20/200 at the 10-year mark. The cause of vision loss was attributed to progressive chorioretinal atrophy surrounding previous sites of CNV.4
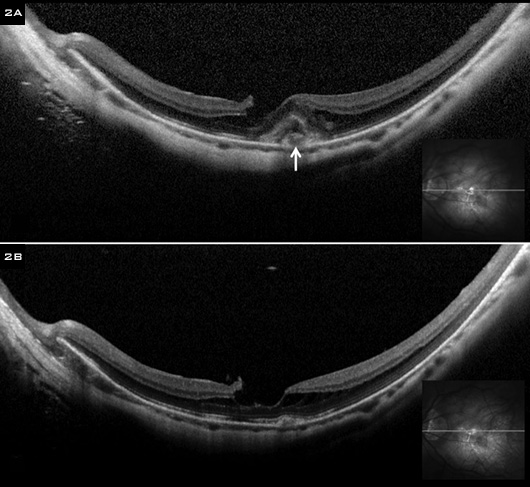 |
|
RESPONSE. (2A) SD OCT images of the same patient demonstrate foveal retinoschisis and CNV (white arrow). (2B) After a single treatment with intravitreal bevacizumab, the CNV regressed in the course of a month. As of the most recent follow up (two years after the injection), she has not developed new or recurrent CNV.
|
Diagnosis
FA. Fluorescein angiography (FA) may be useful in detecting myopic CNV (Figs. 1C, 1D). Hyperfluorescence, often with a hypofluorescent rim of pigmentation, is seen in early images. In late angiographic images, leakage may be subtle and is characterized by blurring of the pigmented rim.
Although myopic CNV typically does not leak to the same degree as CNV in AMD, leakage may be more pronounced in elderly myopic patients. Often, a rim of subretinal hemorrhage accompanies the development of myopic CNV. In such cases, the hyperfluorescence due to leakage by CNV may be blocked by the hemorrhage, making it difficult to discern CNV leakage in the late angiographic images.
Nonneovascular angiographic features in the prearterial phase of the myopic fundus include more visible detail of choroidal vessels due to thinning of the overlying RPE. During FA, an overall decrease in contrast is seen as a result of increased reflection from the underlying sclera, as well as a transmission defect in the early and mid phases and staining of sclera in the late phases. Areas of retinal atrophy may appear as well-defined patches of transmission defect with clearly discernible choroidal vessels. Staining of the sclera is readily noticeable in these atrophic areas. FA can be helpful in detecting lacquer cracks, which appear as hyperfluorescent streaks of transmission defect in the early and transit phases.1
OCT. Spectral-domain optical coherence tomography (SD-OCT) is an essential tool in the evaluation of myopic CNV, which may appear as a hyperreflective lesion with fuzzy borders at the RPE, central ellipsoid, and external limiting membrane (Fig. 2A). Unless subretinal hemorrhage is present, the remainder of the overlying retina may be only minimally elevated or thickened. Intraretinal fluid, sub-retinal fluid, or an RPE detachment may be present, but often these findings are minimal or absent.5
|
Web Extra: Pearls
|
 |
Management
Treatment of degenerative myopia has shifted significantly over the last two decades.
Laser. Although laser photocoagulation has been used for the treatment of CNV related to myopic macular degeneration, it has fallen from favor in the last 15 years. Initially, while significant benefit was seen two years after treatment, the benefit was lost after five years; and high rates of recurrent CNV were noted in treatment groups.6 In many cases, recurrent CNV arose at the margins of previous areas of photocoagulation, suggesting that photothermal disruption of the RPE and Bruch’s membrane may stimulate the development of CNV in some patients. In addition, new lacquer cracks have been reported after photocoagulation, and this may predispose patients to recurrent CNV.
Finally, expansion of the chorioretinal photocoagulation scar has been noted in nearly all highly myopic eyes that undergo photocoagulation. Laser scar expansion may develop quickly and be strikingly broad, and it may expand to involve the fovea and cause significant vision loss.
PDT. Because of the complications associated with laser photocoagulation, photodynamic therapy (PDT) emerged as a treatment for myopic CNV. The utility of PDT was supported by the Verteporfin in Photodynamic Therapy (VIP) study, which evaluated patients with subfoveal CNV due to pathologic myopia. The primary outcome of the study was the proportion of eyes that lost fewer than 8 letters of visual acuity during two years of treatment. In this study, 64 percent of treated eyes lost fewer than 8 letters, compared with 49 percent of eyes in the placebo group.7
With regard to longer-term results, a retrospective study of 43 eyes that had been treated with PDT found that visual acuity was stable during the first year but tended to worsen during the second year and thereafter, reaching a loss of nearly 3 lines at seven years. Macular chorioretinal atrophy developed in 83 percent of the patients at the five-year mark.8
Anti-VEGF agents. Over the last decade, intravitreal anti-VEGF therapy has emerged as a promising treatment modality for myopic CNV. Typical response includes resolution of retinal fluid and regression of the subretinal fuzzy lesion as visualized on SD-OCT4 (Fig. 2B).
Short-term results with anti-VEGF therapy are encouraging. However, as this treatment modality is relatively new, long-term results regarding its effectiveness and its effects on visual acuity are needed.9 Moreover, the clinician should be aware that this represents an off-label use of these medications.
If patients with myopic CNV are aware of intravitreal anti-VEGF therapy for neovascular AMD, they may fear a long course of numerous injections. However, current data indicate that treatment of myopic CNV may differ from that of AMD in that fewer intravitreal injections appear to be needed (see “Frequency of Intravitreal Injections”).
Follow-up. Ongoing monitoring by an ophthalmologist continues to be an important aspect of management, as recurrent CNV and chorioretinal atrophy may develop.
___________________________
1 Ryan SJ et al. Retina. 5th edition. Philadelphia: Elsevier; 2012:1115-1133.
2 Hayashi K et al. Ophthalmology. 2010; 117(8):1595-1611.
3 Grossniklaus HE, Green WR. Retina. 1992;12(2):127-133.
4 Yoshida T et al. Ophthalmology. 2003; 110(7):1297-1305.
5 Introini U et al. Eye (Lond). 2012;26(7): 976-982.
6 Soubrane G et al. Bull Soc Ophtalmol Fr. 1986;86(3):269-272.
7 Blinder KJ et al. Ophthalmology. 2003; 110(4):667-673.
8 Giansanti F et al. Retina. 2012;32(8):15471552.
9 Oishi A et al. Graefes Arch Clin Exp Ophthalmol. 2013;251(1):1-7.
___________________________
Dr. Raecker is a fellow in ophthalmology at the Department of Ophthalmology and Visual Sciences at the University of Iowa, Iowa City. Dr. Park is a resident in ophthalmology, and Dr. Lauer is professor of ophthalmology, vice-chair for education, residency program director, and chief of the Vitreoretinal Division; both are at the Casey Eye Institute at Oregon Health & Science University, in Portland, Ore. Financial disclosure: The authors report no related financial interests. This article was supported by unrestricted departmental funding from Research to Prevent Blindness in New York and the Ophthalmology Resident Education Fund at Casey Eye Institute. The funding organizations had no role in the design or conduct of this article.