Download PDF
In a small proof-of-concept study, researchers at the University of California, San Francisco (UCSF) have confirmed that metagenomic deep sequencing (MDS), which comprehensively samples all genomes in a clinical specimen, can be used to enhance clinicians’ ability to diagnose corneal infections.1
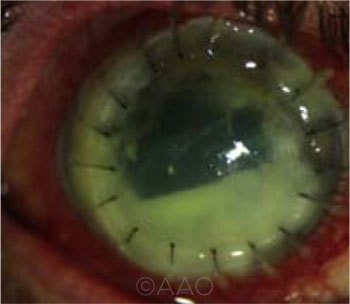 |
UNEXPECTED FINDING. In this patient, MDS detected Auricoccus indicus, which is not known to be associated with ocular infections, as well as Purpureocillium lilacinum.
|
Study specifics. Researchers in the UCSF lab of Thuy Doan, MD, PhD, set out to compare MDS with standard microbiologic testing for diagnosing corneal, scleral, and conjunctival infections in nine patients. MDS was able to identify all disease-causing organisms, whether they were of parasitic, fungal, bacterial, or viral origin.
“A traditional culture favors the known organisms; MDS may be better for unexpected or atypical infections,” said Gerami D. Seitzman, MD, at UCSF. “The sequence information can give us not only the name of the organism but also outline its antibiotic susceptibility.”
Barriers to use. “MDS for ocular infections is still in an experimental phase; to use it, you have to be part of a treatment trial. At the present time, there aren’t any CLIAA-certified labs using it as a formal diagnostic test specifically for the eye,” Dr. Seitzman said.
Additional challenges include the following:
Cost. Currently, the researchers said, “the base reagent and sequencing cost of MDS for a single patient (two swabs each) range from $300 to $1,000 depending on the extent of parallel library processing and the type of sequencing machine used.”1
“Overall, MDS will be several times more expensive” than a standard culture, Dr. Seitzman acknowledged. “However, it becomes more cost-effective for treatment-resistant cases—that is, in cases where the diagnosis is unknown and the disease progresses.” In these instances, she noted, “We often perform numerous repeat cultures.”
Cross-contamination. This may prove to be the biggest challenge to solve. As Dr. Seitzman pointed out, “There is so much DNA in the air or [on surfaces] in a treatment room. MDS is such a sensitive test that may be prone to cross-contamination, even from just talking during the swabbing.”
Bottom line. “As the technology improves, the cost will become lower and our bioinformatic algorithms will continue to improve—and so will our ability to differentiate causative organisms from flora and background,” Dr. Seitzman said.
Eventually, it may be that MDS will not be needed to identify typical community-acquired organisms and will find its greatest benefit at referral centers where more complex or treatment-resistant ocular infections are often seen. That was the instance in this investigation, she noted. “Essentially, this study selected for unusual cases; these patients were previously treated and were referred to us because they were not doing well.”
—Jean Shaw
___________________________
1 Seitzman GD et al. Ophthalmology. 2019;126(12):1724-1726.
___________________________
Relevant financial disclosures—Dr. Seitzman: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Choritz Nonfinancial support for other clinical studies from Allergan, Novartis, and Santen.
Dr. Jonker None.
Dr. Patel None.
Dr. Seitzman Dompé: C.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review