By Sanjay G. Asrani, MD, with L. Jay Katz, MD, Michael S. Kook, MD, and Kazuhisa Sugiyama, MD, PhD
Download PDF
Normal-tension glaucoma (NTG) is a challenging condition with many nuances in diagnosis, monitoring, and treatment. In this first installment of a 2-part series, Sanjay G. Asrani, MD, of the Duke Eye Center in Durham, North Carolina, hosts an MD Roundtable with L. Jay Katz, MD, of the Wills Eye Hospital and Thomas Jefferson University in Philadelphia, Michael S. Kook, MD, of the University of Ulsan and the Asan Medical Center in South Korea, and Kazuhisa Sugiyama, MD, PhD, of Kanazawa University in Japan. The experts discuss the various presentations of NTG and give tips on differential diagnosis. Part 2 of this series, which addresses management of NTG, will appear in the July EyeNet.
The Many Definitions of NTG
Dr. Asrani: How do you define NTG in your clinical practice?
Dr. Sugiyama: We define NTG as optic neuropathy that progresses despite nonmedicated intraocular pressure (IOP) of less than 22 mm Hg. Otherwise, the clinical features of NTG are similar to those of primary open-angle glaucoma. About 70% of patients with glaucoma in Japan have the normal-tension type.1
A challenge in diagnosing NTG is that it is nearly impossible to know if a patient’s IOP is normal all the time. Rather than obtaining diurnal measurements, IOP typically is determined only during office hours. Therefore, instances of high IOP may be missed.
Dr. Kook: Traditionally, we define NTG as glaucoma with an IOP of 21 mm Hg and under. This IOP threshold is based on results of a 1958 population-based study using the Schiotz tonometer and a 2-standard-deviation cutoff.2 However, this definition has been subject to question, as IOP level may differ in other populations and when using different tonometers. For example, we demonstrated recently that the mean IOP in a healthy population from the Namil-myon area of South Korea was 13.3 ± 2.7 mm Hg using the Goldmann applanation tonometer.3 Based on a cutoff of 2 standard deviations from the mean, the threshold for NTG would be an IOP of 18 or 19 mm Hg. I am not sure that defining NTG based on IOP level is clinically helpful. Instead, I find it more useful to differentiate glaucoma based on the underlying risk factors such as IOP-dependent versus non-IOP–dependent type.
Dr. Katz: We apply a similar cutoff to define NTG; we’ve traditionally used IOP values between 21 and 23 mm Hg in the United States, based on the 2-standard-deviation rule from a mean IOP of 16 mm Hg. However, if we regard NTG as primary open-angle glaucoma that is at least partially dependent on IOP, then we should acknowledge that these cutoffs represent only a limited statistical definition.
There are some sources of measurement error in determining a patient’s IOP, such as error associated with corneal thickness. As Dr. Sugiyama noted, we also can miss elevated pressures; study findings have shown that approximately half of peak IOP levels occur outside of typical office hours.4-6
We still apply an IOP cutoff to define NTG, but keep in mind that we manage NTG much as we would high-tension glaucoma: by lowering IOP.
 |
|
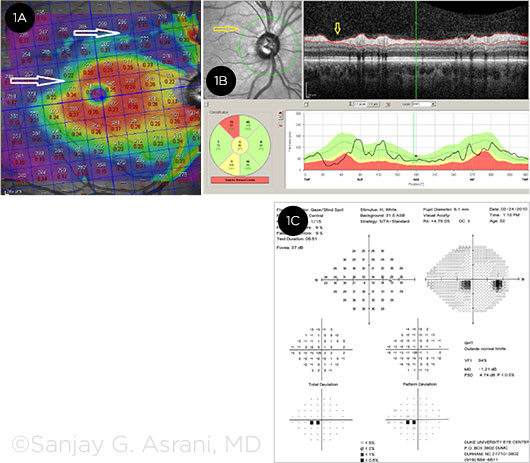
SEVERAL TESTS. (1A) The OCT macular thickness map of this patient shows the focal but deep loss of thickness superiorly, along with early affliction of the parafoveal ganglion cell thickness (2 white arrows). (1B) The OCT retinal nerve fiber layer of the same eye shows focal deep loss in the superotemporal area seen in the cross section as well as in the red-free image (yellow arrows). (1C) The visual field of the same eye shows early inferior paracentral scotoma.
|
Know the Presenting Signs
Dr. Asrani: Which presenting features support the diagnosis of NTG?
Dr. Asrani: As an example, NTG is associated with Flammer syndrome, which involves a constellation of symptoms related to reactive blood vessels and may include difficulty falling asleep, being a high achiever, or having low body mass index (BMI). Other conditions that often accompany NTG are low blood pressure, migraine, and Raynaud syndrome.
Dr. Katz: It can be helpful to assess the patient’s family history. The results won’t necessarily distinguish normal-tension from high-tension glaucoma, but it’s a good starting point. I agree that vasospastic disease, such as migraine or Raynaud syndrome, often is associated with NTG. Vasculopathy also seems to correlate with this disease.
The effects of NTG on the visual field (VF) and optic nerve seem to be more focal in NTG than in high-tension glaucoma. For example, with NTG, we see more focal notching, acquired pits of the optic nerve, and disc hemorrhages. And the initial visual field deficits in patients with NTG tend to be denser and perhaps closer to fixation than would be expected in high-tension glaucoma. However, these are trends and not exclusive rules.
Dr. Kook: I’ve found that associations of NTG with some clinical features such as Flammer syndrome may vary between populations. In Korea, for instance, migraine may not be as common as Raynaud syndrome or nocturnal hypotension in patients with NTG. In NTG patients with disease progression despite well-controlled IOP, it is of importance that the clinician rule out nocturnal hypotension due to either primary vascular dysregulation or secondary to overtreatment with antihypertensive medications. Nocturnal hypotension, particularly associated with overtreatment of systemic hypertension, can be alleviated or prevented in close collaboration with the internist.
In agreement with Dr. Katz, I frequently note focal features in NTG, such as a focal defect in the neuroretinal rim or optic disc hemorrhage that is confined to the inferotemporal or superotemporal region. Cupping in the optic disc tends to be shallow and sloped rather than deep and steep in appearance, and the visual field defects in NTG may often involve the central or paracentral region in the superior hemifield area.
We can attempt to differentiate NTG from primary open-angle glaucoma based on optic nerve appearance or systemic features—although I think it can be quite challenging, as NTG may be on a spectrum of disease having IOP and/or non-IOP risk factors. Some patients with NTG may have clinical features that are very consistent with primary open-angle glaucoma (POAG).
Dr. Sugiyama: We see many NTG patients who have sleep apnea. They may have high spikes in IOP and also have hyper- or hypotension at night.
In my experience, several features are more common in NTG than in POAG. Disc hemorrhage is common in NTG and is a sign of disease progression. This hemorrhage tends to involve a wedge-shaped defect of the retinal nerve fiber layer (RNFL). I have found that NTG also is associated with peripapillary atrophy of the beta zone.
Recognize Masqueraders
Dr. Asrani: Several conditions can masquerade as NTG. Which clinical features make you doubt that it is NTG?
Dr. Sugiyama: The disc shape in superior segmental optic hypoplasia (SSOH) is similar to that in NTG. Moreover, both conditions involve focal hypoplasia of the nasal superior sector of the disc and often include a lower visual field defect.
Dr. Kook: I agree. SSOH is one of several congenital optic disc anomalies that exhibit localized optic disc changes and present like glaucoma in terms of an RNFL or visual field defect. Others include optic disc drusen, optic nerve pits, and optic nerve hypoplasia.
Myopia is another common condition that can have a presentation similar to glaucoma in terms of clinical features including optic disc and/or RNFL defects or VF deficits respecting the horizontal midline. However, while glaucoma is a progressive optic neuropathy, the natural course of myopia may be different. Optic disc and/or visual field changes associated with myopia may not progress after myopic developmental changes cease in young individuals with a normal IOP level. Optic disc features that are often associated with these young individuals may include optic disc tilt or torsion. In the clinical setting, we should follow up regularly and monitor the glaucomatous-appearing optic disc changes or VF defects found in myopic eyes that may progress over time due to glaucoma or remain nonprogressive due to myopic developmental findings.
Clinical examination of the optic disc is the most important way to differentiate nonglaucomatous masqueraders from NTG. Careful ocular examination can help us determine whether the optic disc has what we call “compatible” glaucomatous cupping, which may traditionally be defined as increased generalized cupping with vertical cup-to-disc ratio greater than 0.7, and focal loss of the neuroretinal rim with accompanying RNFL defects. For example, vascular changes in the retina, such as hemiretinal or branch retinal vein occlusion, can masquerade as glaucoma in terms of VF changes but do not involve cupping; these retinal vascular abnormalities eventually yield arteriosclerotic vessels.
Imaging modalities such as optical coherence tomography (OCT) also can help us differentiate certain congenital anomalies from glaucoma. Imaging findings that indicate glaucoma may include characteristic superotemporal and/or inferotemporal arcuate areas of pathogenicity. In contrast, findings that suggest congenital optic nerve anomalies often involve thinning of the temporal or nasal side of the RNFL or neuroretinal rim. Inflammatory conditions, including optic neuritis and anterior ischemic optic neuropathy, may also masquerade as glaucoma in terms of clinical presentation but entail lesions of the temporal or nasal side of optic disc in imaging studies.
Dr. Asrani: When we use OCT, we look for arcuate patterns closer to the fovea in the macular thickness loss in NTG; the arcuate shape is characteristic of glaucoma rather than the masquerading conditions.
Dr. Katz: On examination, it’s important to note whether there’s asymmetry in the cupping and corresponding asymmetry in the IOP. If so, I would feel comfortable with a glaucoma diagnosis even without strong evidence of visual field loss because structural ocular changes often precede functional ones.
We don’t want to miss serious issues that mimic glaucoma. Certain findings would be atypical for NTG in the United States: young age; central visual acuity losses; a cecocentral scotoma, rather than the arcuate or paracentral defects more typical of glaucoma; visual field loss that respects more of the vertical midline; and optic nerve pallor greater than cupping. Such findings might prompt further workup, including magnetic resonance imaging of the head and orbits to detect etiologies involving the central nervous system (CNS).
We also would consider optic neuropathies unassociated with IOP, such as those due to toxic, drug-related, or nutritional conditions. We used to perform imaging of the head frequently to evaluate NTG. Today, imaging is reserved for these more atypical features—with CNS imaging, we tend to find in patients with typical features of glaucoma only small vessel disease indicative of mild systemic vasculopathy.
A clinical exam is vital and can reveal findings that can’t be obtained with imaging alone. The retina and optic nerve should be examined carefully. You should evaluate pallor in the optic nerve; if the amount of pallor exceeds the amount of cupping, the etiology may be nonglaucomatous. You should also consider the vasculature, as small branch retinal vein occlusion, embolic plaques, or a slowly advancing retinal detachment can masquerade as progressive visual field change.
Certain blood tests can be performed to rule out masquerading conditions. If we find pallor of the optic nerve during clinical examination, we usually order a complete blood count to test for profound blood loss or anemia. Hypotension combined with anemia can result in ischemic changes. In a workup for inflammatory diseases that may mimic NTG in presentation, I would determine the erythrocyte sedimentation rate and the level of C-reactive protein.
Dr. Asrani: In terms of blood tests, I typically order the fluorescent treponemal antibody absorption test (FTA-ABS) for neurosyphilis. I also request measurement of B12 levels because a deficiency of vitamin B12 sometimes masquerades as NTG.
Dr. Sugiyama: In Japan, high myopia is prevalent and involves a disc shape akin to that of NTG. We have to be careful in the differential diagnosis, and visual field testing is crucial for this.
Patients with NTG usually have upper or lower visual hemifield defects and the fixation point is spared until late stage. In patients with high myopia and glaucoma, the fixation point is often affected at an early stage of visual field loss; frequently, the first obvious stage that we observe is deep scotoma close to the fixation point. We call this an early-stage central visual field loss. Close monitoring of these patients is key.
Dr. Asrani: A typical feature of NTG is paracentral visual field loss that does not involve central acuity. I find that OCT results of patients with NTG indicate arcuate patterns accompanied by losses of paracentral ganglion cell groups—these losses occur at an earlier stage than in high-pressure glaucoma.
 |
|
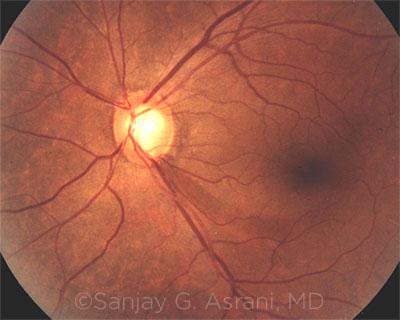
FUNDUS PHOTO. This image shows a focal loss of the retinal nerve fiber layer inferiorly, extending into the parafoveal region (dark band extending from disc at 5 o’clock position).
|
Consider Systemic Factors
Dr. Asrani: Have you noticed an association of low body mass index and/or low cerebrospinal fluid (CSF) pressure and NTG?
Dr. Kook: Patients diagnosed with NTG often have low BMI and low CSF pressure. Dr. Ningli Wang and colleagues demonstrated an association of CSF pressure with IOP in NTG.7 Their work was based on the hypothesis that pressures in the arterial system, CSF compartment, and intraocular space are related. Theoretically, low CSF pressure could produce an elevated translaminar pressure gradient and result in NTG despite normal IOP. However, I don’t know the frequency of this phenomenon, and I do not presently use this concept in clinical practice. Moreover, currently, there is no gold standard for accurately measuring CSF pressure as well as IOP in our habitual-body position.
Dr. Katz: The idea that people who have high BMI are more protected from glaucoma is interesting to me, but I wouldn’t incorporate this into clinical practice. The association of low BMI and NTG underscores the complexity of this disease. We know that IOP is important, but there are additional contributors. The translaminar pressure gradient seems to be another stress factor involved in development of NTG. However, it’s challenging to measure CSF pressure accurately, and even if we can determine this pressure, how would we change therapy?
Blood pressure seems to be another pressure factor in NTG. Glaucoma prevalence is higher among patients with low diastolic blood pressure.8 These 3 pressure components might be important in the development of NTG and may have contributions that vary on an individual level.
Another potential component is metabolic dysfunction—whether mitochondrial or otherwise. A metabolic abnormality could result in excessive cell death signals and/or a lack of cell-survival signals that could lead to death of retinal ganglion cells.
Other Diagnostic Enigmas
Dr. Asrani: I have treated patients with NTG who subsequently develop high-pressure glaucoma. In these patients, the glaucoma suddenly and dramatically worsened. During examination, I have observed intermittent angle closure or chronic angle closure in many of these patients. These conditions cause high spikes in IOP. Even if you treat the compounding problem by cataract extraction or laser iridotomy, the residual NTG remains.
Dr. Katz: Glaucoma has several diagnostic enigmas. One is burned-out pigmentary glaucoma, in which the patient loses some pigmentary dispersion features but no longer has elevated IOP.
Some patients may have had steroid-induced pressure elevation, but by the time you see them, they are off the steroids; they have definite optic nerve deterioration and visual field loss, but IOP is no longer elevated.
Dr. Sugiyama: I agree with Dr. Asrani; intermittent acute angle-closure glaucoma is an important consideration in the differential diagnosis of NTG. Another problem of measuring IOP is corneal thickness. If the center of the cornea is very thin, this can result in underestimation of IOP.
Another diagnostic challenge relates to Posner-Schlossman syndrome. These patients can present with NTG but also may have spikes in IOP.
___________________________
1 Iwase A et al. Ophthalmology. 2004;111(9):1641-1648.
2 Leydhecker W et al. Klin Monbl Augenheilkd. 1958;133(5):662-670.
3 Kim CS et al. Ophthalmology. 2011;118(6):1024-1030.
4 Liu JH et al. Invest Ophthalmol Vis Sci. 2003;44(4):1586-1590.
5 Barkana Y et al. Arch Ophthalmol. 2006:124(6):793-797.
6 Nakakura S et al. J Glaucoma. 2007;16(2):201-204.
7 Wang N et al. Ophthalmology. 2012;119(10):2065-2073.
8 Mitchell P et al. Am J Ophthalmol. 2005;140(1):131-132.
___________________________
Dr. Asrani is professor of ophthalmology at the Duke Eye Center of Durham, N.C. Relevant financial disclosures: None.
Dr. Katz is the director of the Glaucoma Service of the Wills Eye Center and professor of ophthalmology at the Thomas Jefferson University in Philadelphia. Relevant financial disclosures: None.
Dr. Kook is professor of ophthalmology at the University of Ulsan College of Medicine and in practice at the Asan Medical Center, both in Seoul, South Korea. He is also president of the Korean Glaucoma Society. Relevant financial disclosures: None.
Dr. Sugiyama is professor and chairman of the Department of Ophthalmology at Kanazawa University in Japan. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Asrani Camras Vision: C; Noveome Biotherapeutics: C.
Dr. Katz Aerie: C,L,O; Aerpio: C; Alcon: C,L,S; Allergan: C,L,S; Bausch + Lomb: L; Diopsys: C,S; Glaukos: C,E,L,O; Heidelberg: S; Mati Therapeutics: C,O,S.
Dr. Kook None.
Dr. Sugiyama Alcon: L,S; Kowa: C,L,S; Nidek: L,S; Santen: C,L,S; Senju: C,L,S; Topcon: L,S.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|