Download PDF
How can visual gains achieved in a clinical trial be sustained once patients enter the real world of standard clinical care? In an extension of the two-year DRCR.net’s Protocol T study, anti-VEGF treatment improved vision over five years in eyes with visual acuity (VA) impairment from diabetic macular edema (DME). But some of the gain at the two-year mark was lost when patients left the trial setting.1
A previous DRCR.net study, Protocol I, found that VA was maintained through five years when a structured protocol was followed.2 “We were hoping that the visual acuity results in Protocol T would parallel the prior study and show stability in vision between two and five years,” said Adam R. Glassman, MS, at the Jaeb Center for Health Research in Tampa, Florida.
Does that mean that something happens when patients are no longer followed in a rigorously controlled setting? “That’s speculation,” said Mr. Glassman. “But it’s not an unreasonable speculation.”
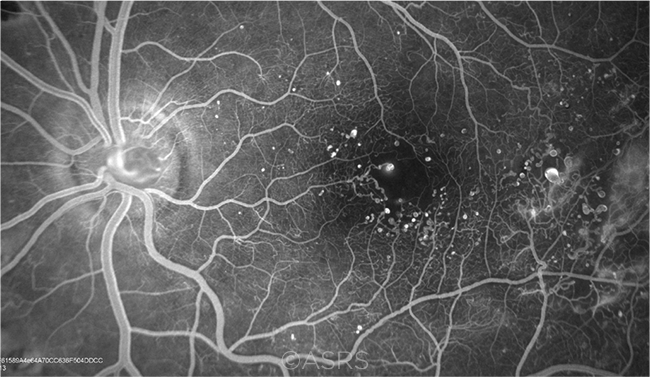 |
DME. Fluorescein angiogram shows DME, microaneurysms, and neovascularization in a 39-year-old patient with long-standing diabetes. This image was originally published in the ASRS Retina Image Bank. James B. Soque, CRA, OCT-C, COA. Diabetic Macular Edema, Proliferative Diabetic Retinopathy, Neovascularization Elsewhere. Retina Image Bank. 2012; Image Number 5480. © The American Society of Retina Specialists.
|
The initial study. For Protocol T, 660 diabetic adults at 88 sites were randomized to receive aflibercept, bevacizumab, or ranibizumab as first-line treatment for visual impairment from center-involved DME. Visits were scheduled every four weeks in year 1 and every four to 16 weeks in year 2, depending on treatment response.
The extension. For the next three years, 317 (68%) of 463 eligible patients received standard care and were evaluated at the five-year mark.
During the three-year extension, 95% had at least one office visit with a retina specialist. The median number of visits for years 3, 4, and 5 were four, three, and four, respectively. (In contrast, the median number of visits was nine in year 2.)
In addition, 68% of patients in the extension study received at least one anti-VEGF treatment, with a median of four injections. The choice of anti-VEGF agent during the first two years did not lead to any statistically significant treatment group differences in VA at five years.
At the five-year mark, 30% gained 7.4 letters from baseline, but mean VA worsened by 4.7 letters from the two-year assessment. All told, nearly half of eyes (47%) were 20/25 or better and 5% were 20/200 or worse at five years.
A surprise finding. Mean central subfield thickness decreased from baseline by 154 μm and remained stable throughout five years despite the fact that average VA worsened during the extension study. “The reasons for this are unclear, but this finding highlights the importance of evaluating both anatomic and functional results in eyes with DME,” Mr. Glassman said.
Ongoing challenge. Once a trial has ended, how can visual gains be sustained? “This is a challenging issue, since there are so many variable factors in clinical practice that are controlled in clinical trials,” Mr. Glassman said. Future studies might explore barriers to clinical care, he added. In the meantime, he advised teaching patients the importance of regularly scheduled retinal exams, even if they are not experiencing visual symptoms.
—Miriam Karmel
___________________________
1 Glassman AR et al. Ophthalmology. Published online March 28, 2020.
2 Elman MJ et al. Ophthalmology. 2015;122(2):375-381.
___________________________
Relevant financial disclosures—Mr. Glassman: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Feng None.
Dr. Gibbons None.
Mr. Glassman None.
Dr. Gupta None.
Dr. Koch Alcon: C; Carl Zeiss Meditec: C; CapsuLaser: O; Johnson & Johnson Surgical Vision: C; Ivantis: O; Perfect Lens: C; Vivior: O.
Dr. Leung None.
Mr. Robbins None.
Dr. Wygnanski-Jaffe GoCheck Kids: C; NovaSight: C.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review