Download PDF
Cytomegalovirus retinitis is the most common ophthalmic opportunistic infection in patients with acquired immunodeficiency syndrome (AIDS),1 and the condition can have devastating consequences for vision. One study showed the cumulative incidence of bilateral vision impairment at 10 years following diagnosis of cytomegalovirus retinitis (CMVR) to be 11.2%.2 Despite decreased rates of CMVR with highly active antiretroviral therapy (HAART), treatment-resistant CMVR remains a particular challenge.
Epidemiology
Before the era of HAART, the rate of CMVR in persons with AIDS was estimated to be 30%. Rates have significantly decreased since the advent of HAART; one study found that the 10-year cumulative incidence of CMVR was 4.25%.3 However, the disease remains a concern in AIDS patients with inadequate immune recovery or resistance to treatment, as well as in immunosuppressed organ transplant patients without AIDS.
Drug-resistant CMVR has proved to be another challenging issue confounding treatment, particularly in AIDS patients. Studies have shown that after nine months of treatment for CMVR, resistance to ganciclovir (GCV) and foscarnet (FOS) increased from 1% and 2% to 27% and 37%, respectively.4
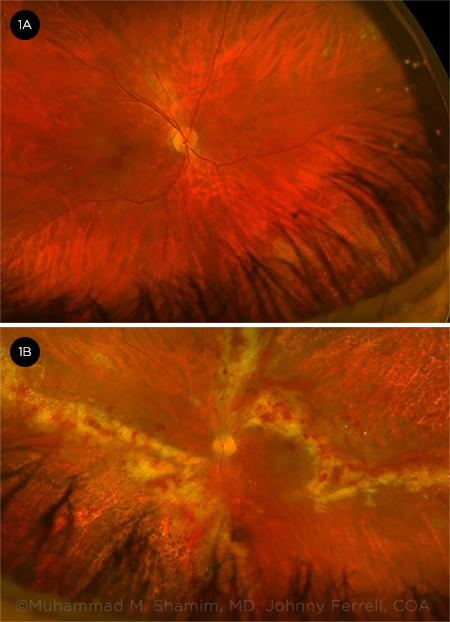 |
|
PRESENTATION. (1A) Suspicious lesion in the right eye. (1B) Retinitis and retinal hemorrhages in the left.
|
Pathophysiology
CMV is a double-stranded DNA virus in the Herpesvirus family, which exists within a protein capsid with a lipid bilayer. These structures allow the virus to invade the host cells for replication, after which CMV can establish latency in both the blood and the bone marrow.1 Subsequently, viral reactivation can occur in individuals with weakened immune systems, such as those with HIV infection or organ transplant recipients on immunosuppressive therapy.1 In the eye, uncontrolled CMV replication within vascular endothelial cells and retinal pigment epithelial cells leads to retinal tissue necrosis, retinal detachment, and blindness in severe cases.1
Several risk factors for the development of drug-resistant CMVR have been described, including drug exposure for more than three months, high peak viral load, recurrent infection, and T-cell depletion.5 Resistance can be categorized as laboratory-confirmed resistance or clinical resistance in the absence of known mutations. Of the genetic etiologies for resistance, mutations in the phosphotransferase gene (UL97) or the viral polymerase gene (UL54) have been studied the most, although new mutations with varying effects on resistance are continually being discovered.5
Clinical Features and Diagnosis
Patients with symptomatic CMVR most commonly report blurred vision, while other symptoms include floaters or scotomas. However, in most infected patients, CMVR is asymptomatic and found incidentally.
Features. The clinical features of CMVR can vary depending on the reason for the immunocompromised state. In HIV-negative patients, such as transplant patients, clinical features include higher-than-normal rates of retinal arteritis and vitritis, as well as vascular occlusion extending beyond the areas of retinitis. In HIV-positive patients, the two most common forms of CMVR are indolent and fulminant. The indolent form is characterized by white, granular, progressive necrotizing retinitis in the periphery with little or no hemorrhage. In the fulminant form, there is retinal hemorrhage with full-thickness yellow-white lesions in a perivascular arrangement.
Severity. The clinical severity of CMVR can be further determined by the zonal location of the lesions. Zone 1 lesions are located within 1 disc diameter (DD) of the disc and 2 DD around the fovea; these lesions are considered sight-threatening. Zone 2 lesions are located anterior to zone 1 and posterior to the vortex vein ampullae, while zone 3 lesions are located peripheral to zone 2.
Diagnosis. The diagnosis of CMVR is mainly a clinical one based on presence of the typical pattern of retinitis outlined above. In order to confirm diagnosis, and in cases where clinical features are atypical, polymerase chain reaction (PCR) analysis of ocular fluid may be performed.1
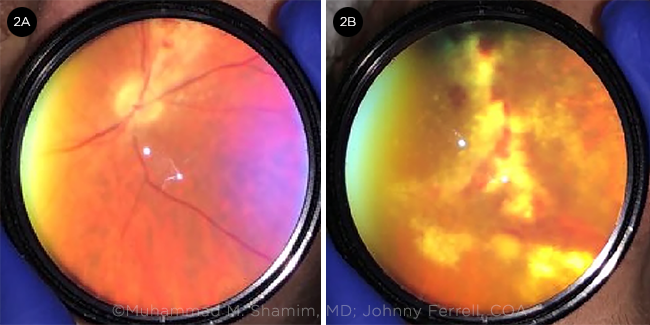 |
|
AT ONE WEEK FOLLOW-UP. Fundus photographs captured at bedside with smartphone and 20D lens. (2A) Progression of CMVR in the right eye. (2B) Progression of CMVR in the left eye.
|
Management
High-dose induction antiviral therapy for two to three weeks is started in patients when active CMVR is found.1 However, patients should be monitored every two or three days to evaluate the progression of the disease. The ultimate duration of induction therapy is determined based on disease progression or resolution. In HIV patients, induction therapy is followed by maintenance therapy until the CD4 count increases to more than 100 cells/uL for more than six months.
Agents. There are three routes of drug administration for CMVR management: intravenous, oral, and intravitreal injections. Systemic GCV and valganciclovir (VGCV) constitute the first-line treatment for CMVR, with FOS and cidofovir (CDV) being used as a second-line agents. GCV, FOS, and CDV can also be administered by intravitreal injection, while VGCV is only available in oral form.5
Administration. The choice of treatment modality in CMVR depends on the location of the lesions, as well as other factors such as side effects and the patient’s adherence to therapy.1 If lesions are present in zone 1, intravitreal injections are preferred, in combination with systemic therapy. In patients without immediately vision-threatening lesions, systemic therapy is recommended, along with close observation. For those with refractory retinitis, combination therapy with systemic GCV and FOS is recommended.
An implanted device consisting of a pellet of GCV in a biocompatible polymer (Vitrasert, Bausch & Lomb) was formerly available. It allowed for local, sustained intraocular release of GCV in patients with CMVR, who often could not tolerate the systemic side effects of the medication. However, this device was discontinued in 2013.
Drug-Resistant CMVR
It has been shown that treatment of unilateral CMVR with systemic GCV results in a higher rate of drug resistance in contralateral eyes that subsequently develop CMVR.6 In systemic CMV disease, drug resistance is suspected when patients have received two weeks of antiviral therapy without improvement in CMV viremia (>1 log10 increase in CMV DNA levels in serum) or with the onset or progression of end-organ disease after at least six weeks of antiviral treatment.
Rapid progression. In the setting of drug-resistant CMVR, clinical progression to zone 1 disease may be rapid and necessitate earlier-than-normal changes in the treatment regimen to ensure that lesions regress or at least stabilize. Serial eye exams, done every three or four days, should be performed when CMVR involves zone 1.
Treatment options. In cases with low-level GCV resistance, combined systemic GCV/FOS may be a viable treatment option. Additionally, systemic adjuvant therapy with leflunomide has been shown to be efficacious in drug-resistant CMV disease, although toxicity may limit its use.7 More recent options being evaluated for the management of drug-resistant and refractory systemic CMV disease include modulating immune function with mTOR inhibitors or CMV intravenous immunoglobulin, as well as novel antiviral therapies such as maribavir, brincidofovir, and letermovir. These drugs have been shown to have high potency against multidrug-resistant CMV. However, their use in CMVR specifically has not been established.5
Testing for resistance. Laboratory confirmation of CMV drug resistance based on genotypic analysis of specimens may be of use in tailoring the treatment regimen when resistance is clinically suspected. The literature suggests that the patient should complete two weeks of full-dose antiviral therapy and at least six weeks of total therapy before genotypic testing in most cases.8
CMV load has also been shown to be associated with CMVR progression and occurrence of resistant CMVR. Although the clinical utility of CMV load for prediction of resistance is limited by its low positive predictive value, the negative predictive value of CMV load for resistance is excellent. Thus, measurement of CMV load may be useful in rapidly excluding resistance and in screening patients who would benefit from more detailed resistance testing.9
Genotypic testing is done on blood or plasma specimens, although it is important to note that at the time of presentation of CMVR, the virus may not be detectable in the blood or tissue.10 In these cases, clinical suspicion of viral resistance may be sufficient. The first mutations to be discovered were those of the phosphotransferase gene (UL97) conferring GCV resistance. Subsequently, mutations in UL54 (viral polymerase gene) were discovered, which conferred cross-resistance to CDV and FOS. Mutations with varying effects on resistance continue to be discovered.
Conclusion
Rates of CMVR in immunocompromised patients have decreased drastically since the advent of HAART. However, patients with treatment-resistant disease pose a particular challenge because of the risk of irreversible vision loss. Thus, screening for resistance may be of benefit to prevent vision loss in some patients. For those with zone 1 involvement or a history of prior treatment with GCV, testing for GCV resistance on presentation is recommended, as is close follow-up with fundus exams and/or photographs performed every three to four days. Alternatively, given the high negative predictive value of CMV load for drug resistance,9 patients may be screened with that method to assess whether they should be tested further for drug resistance.
___________________________
1 Munro M et al. Microorganisms. 2019;8(1):55.
2 Jabs DA et al. Ophthalmology. 2015;122(7):1452-1463.
3 Sugar EA et al. Am J Ophthalmol. 2012;153(6):1016-1024.e5.
4 Jabs DA et al. Antimicrob Agents Chemother. 1998;42(9):2240-2244.
5 Fu L et al. Ocul Immunol Inflamm. 2020;28(7):1152-1158.
6 Imai Y et al. J Infect Dis. 2004;189(4):611-615.
7 Levi ME et al. Transpl Infect Dis. 2006;8(1):38-43.
8 Kotton CN et al. Transplantation. 2013;96(4):333-360.
9 Jabs DA et al; Cytomegalovirus Retinitis and Viral Resistance Research Group. J Infect Dis. 2005;192(4):640-649. [Published correction appears in J Infect Dis. 2005;192(7):1310.]
10 Chou S. Curr Opin Infect Dis. 2015;28(4):293-299.
___________________________
Dr. Shamim is an ophthalmology resident at Jones Eye Institute in Little Rock, Ark. Dr. Uwaydat is a vitreoretinal surgeon at Jones Eye Institute in Little Rock, Ark., and a professor of ophthalmology at the University of Arkansas for Medical Sciences. Financial disclosures: None.
The authors thank Hytham Al-Hindi, fourth-year medical student at the University of Arkansas for Medical Sciences, for his contributions.
Case Study
A 35-year-old man with a history of HIV/AIDS who was taking Biktarvy (a combination of bictegravir, emtricitabine, and tenofovir alafenamide) presented to the emergency department with a two-week history of gradual vision loss in the left eye. He had been diagnosed with HIV infection approximately one year earlier and had been hospitalized multiple times for AIDS-related complications. He had been on oral VGCV 450 mg daily for at least eight months prior to the current presentation for a history of presumed CMV pneumonia, but when we saw him the VGCV had been discontinued for unknown reasons.
Initial exam. On examination, the patient’s best-corrected visual acuity (BCVA) was 20/20 in the right eye and hand motion in the left. Pupil exam demonstrated a 3+ relative afferent pupillary defect in the left eye. Dilated fundus exam in the right eye showed a suspicious lesion superior to the optic nerve, initially thought to be a cotton-wool spot (Fig. 1A). Dilated fundus exam in the left eye was significant for retinitis with retinal hemorrhages involving the inferonasal and superotemporal retina, as well as the superior aspect of the macula (Fig. 1B). Anterior chamber paracentesis was performed, and an aqueous sample was sent for PCR testing for herpes simplex virus, varicella-zoster virus, CMV, and Toxoplasma. Intravitreal GCV (2.0 mg/0.05 mL) was administered to the left eye.
ICU. The patient was admitted to the ICU for treatment of sepsis and acute respiratory failure secondary to pneumonia. Clinicians on the infectious disease service started him on the induction dose of oral VGCV 900 mg twice daily. He was also started on broad-spectrum antibiotics in the setting of sepsis.
GCV-resistant CMVR. The patient was reevaluated in clinic on day 6 of hospitalization, at which time active CMVR was noted in the right eye. PCR testing of the previously submitted aqueous sample confirmed the presence of CMV (4,300,000 IU/mL). Despite continued systemic therapy with oral VGCV, the patient was noted to have progression of CMVR in both eyes (Fig. 2), now involving zone 1 in the right eye. Intravitreal GCV (2.0 mg/0.05 mL) and intravitreal FOS (2.4 mg/0.1 mL) were injected in both eyes. We recommended genotypic testing for GCV resistance at this time. The patient became pancytopenic on VGCV, so he was switched to intravenous FOS treatment. Genotypic analysis of serum CMV detected a mutation in the UL97 gene, with no mutations in the UL54 gene, confirming a GCV-resistant strain of CMV.
For the remainder of the patient’s hospital stay, we continued to treat him with weekly intravitreal FOS only. Upon discharge, BCVA was 20/25 in the right eye and 20/200 in the left. Unfortunately, the patient was lost to follow-up and did not return for weekly intravitreal injections after discharge.
|