By Annie Stuart, Contributing Writer, interviewing Ahmad A. Aref, MD, Harry A. Quigley, MD, and Robert N. Weinreb, MD
NOTE: This article has been corrected since print publication as follows: Rhopressa decreases [rather than promotes] actinmyosin contraction and reduces [rather than increases] actin stress fibers and focal adhesions in the trabecular meshwork to improve the outflow of aqueous humor.
Download PDF
After an extended drought, 2 new—and much anticipated—glaucoma drugs are now on the market. “We’ve had a long stretch without any new glaucoma medications,” said Ahmad A. Aref, MD, at the University of Illinois College of Medicine in Chicago. “Now, at the same time, we have 2 relatively low-risk ways to decrease the threat of irreversible vision loss from glaucoma. That’s a big deal.”
In late 2017, the FDA approved latanoprostene bunod ophthalmic solution (Vyzulta, 0.024%; Bausch + Lomb) and netarsudil ophthalmic solution (Rhopressa, 0.02%; Aerie Pharmaceuticals) for reduction of intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.1,2 “Rhopressa and Vyzulta have stolen the limelight,” said Dr. Aref. “It will be interesting to see how this plays out, especially from a payer perspective.”
Here’s a look at the 2 drugs, plus an update on drugs in the pipeline (see “From Drought to Flood?”).
 |
|
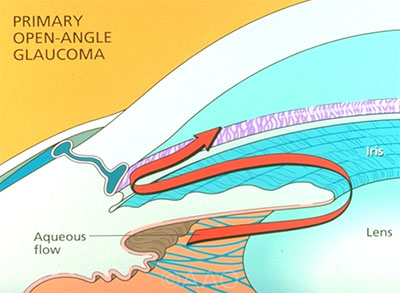
METHOD OF ACTION. Through novel mechanisms, both Vyzulta and Rhopressa improve outflow of aqueous through the trabecular meshwork. Each medication is a once-daily eyedrop.
|
Vyzulta: Releasing Nitric Oxide
A once-daily eyedrop, Vyzulta is a prostaglandin analog that is metabolized into 2 moieties and regulates IOP through both the trabecular outflow and uveoscleral outflow pathways, said Robert N. Weinreb, MD, at the University of California, San Diego.
Dual mechanism of action. “One component is latanoprost, our most efficacious first-line agent for glaucoma, which has been on the market for more than 2 decades and removes fluid through the uveoscleral outflow pathway,” said Dr. Aref.
The second component is butanediol mononitrate, which releases nitric oxide (NO), Dr. Weinreb said. “Nitric oxide induces cell relaxation in the trabecular meshwork by activating the nitric oxide–cyclic guanosine monophosphate signaling pathway, which is thought to lead to a widening of the intercellular spaces in the trabecular meshwork, thereby increasing the conventional outflow.”
The NO component is the unique aspect of the drug’s mechanism of action, giving it a bit of an efficacy edge in lowering IOP over latanoprost alone, said Dr. Aref. When different concentrations of Vyzulta were compared against latanoprost alone,3 only higher concentrations of Vyzulta were found appreciably more effective, he said. “This suggests that the nitric oxide was responsible for the incremental efficacy.”
Efficacy. A veritable space race of studies has examined the effectiveness and safety of Vyzulta. Results of the LUNAR and APOLLO studies showed that Vyzulta was more effective than timolol. Although the findings were not a surprise, the noninferiority study was necessary, said Harry A. Quigley, MD, at the Wilmer Eye Institute in Baltimore. “Before bringing a glaucoma drug to market, the FDA requires that it work at least as well as timolol.”
A study published earlier this year4 also looked at the pooled results of all studies comparing Vyzulta to timolol over 12 months, said Dr. Aref. “With Vyzulta, the percentage reduction in IOP from baseline was 32%. That’s a sizable reduction with just 1 eyedrop dosed once a day.”
The VOYAGER study compared Vyzulta to latanoprost alone. Among the Vyzulta studies, Dr. Aref considers it most significant because latanoprost is the clinical benchmark against which other glaucoma drugs are compared. In this study, Vyzulta was associated on average with 1.2 mm Hg of additional IOP lowering compared to latanoprost.3
“This is fairly significant,” said Dr. Aref, “because epidemiologic studies have shown that for every 1 mm Hg incremental decrease in IOP, you can reduce the risk of visual field loss related to glaucoma by about 10%.”
Other studies have also looked at 24-hour lowering of IOP, said Dr. Weinreb, indicating that Vyzulta is effective both day and night.5
Safety and tolerability. “In a phase 2 clinical trial, Vyzulta was very similar to latanoprost in terms of tolerability,” said Dr. Weinreb. Most side effects, such as irritation and eyelash changes, were mild, and hyperemia was similar in both groups. “But, of course, the drug is only recently available,” he said.
Indeed, wider clinical use may eventually uncover issues with Vyzulta, as has happened with other ophthalmic drugs. Dr. Quigley cited timolol as a case in point: Individuals with dry eyes were not admitted to the study, he said, but once the drug came out of controlled trials into the real world, beta-blockers were found to be challenging for people with dry eyes.
Role for Vyzulta. Vyzulta is appropriate as a first-line treatment option for patients with open-angle [glaucoma] or ocular hypertension, Dr. Weinreb said. Many patients may also be put on Vyzulta as a second-line therapy in an attempt to avoid surgery, Dr. Quigley said. “If you tell patients they have to take this new drug or have surgery, you’ll likely increase their adherence.”
Rhopressa: First ROCK Inhibitor
Like Vyzulta, Rhopressa is a once-daily eyedrop. However, as a Rho kinase (ROCK) inhibitor, it represents the first new class of glaucoma drugs in more than 20 years.
Triple mechanism of action. Rhopressa possesses 3 different mechanisms of action in a single agent, said Dr. Aref. The drug lowers the resistance to outflow through the trabecular meshwork, he said. Rhopressa also decreases production of fluid and decreases episcleral venous pressure.
Among its effects, Rhopressa works at the cellular level within the trabecular network, and it has a novel mechanism of action there, said Dr. Weinreb. “The drug decreases actin-myosin contraction and reduces actin stress fibers and focal adhesions in the trabecular meshwork to improve the outflow of aqueous humor.”
Efficacy. ROCK inhibitors are supported by extensive basic science research showing improvement of outflow through the trabecular meshwork, predominantly for glaucoma patients with higher-than-normal pressures, said Dr. Quigley, but they may also be effective for those with lower pressures. Few studies have examined those with pressures below 20 mm Hg at the time of diagnosis, he said, which is about half of those who have open-angle glaucoma with optic nerve damage.
However, noninferiority timolol studies—ROCKET-1 and ROCKET-2—included lower pressures in their study groups.6 These studies found timolol was not better than Rhopressa for patients with baseline eye pressure less than 25 mm Hg over a 3-month time period, said Dr. Aref. “Rhopressa showed consistent IOP reduction, about 5 mm Hg across a range of baseline pressures,” said Dr. Weinreb, “particularly notable in patients with low baseline IOP.”
Safety and tolerability. In ROCKET-1 and ROCKET-2, about half the patients experienced conjunctival hyperemia, the most common side effect. This redness may result from one of Rhopressa’s mechanisms of action, which is relaxation of the blood vessels, said Dr. Aref.
During the drug’s early days on the market, Dr. Quigley expected reports of redness to be more substantive than those noted during the clinical trials, as trial participants tend to be less tolerant of side effects. “If you have lots of redness in phase 2 and 3 trials, it won’t get better once the drug is used in the real world,” he said.
But now that Rhopressa has been in use for several months, “the redness issues have been much less than what I would have expected from clinical trial data,” Dr. Aref said. “I currently let patients know to expect some degreee of redness that will likely wane over the first few weeks of therapy. That expectation allows patients to tolerate the agent a little better. In practice, it is unlikely for patients to discontinue therapy for this reason alone.”
Other common side effects noted during clinical trials were discomfort with drug administration and conjunctival hemorrhage—typically mild petechiae at the limbus, said Dr. Weinreb. “Twenty percent of patients also experienced corneal verticillata. This side effect does not seem to affect vision and is reversible with discontinuation of the drug.”
Dr. Quigley raised concerns about the safety of drugs like Rhopressa that alter the sclera. Do they have an impact on retinal ganglion cell axons? “It is extremely important to ensure that any negative effect is negligible or that the alteration is potentially beneficial to the ganglion cells,” he said. “However, we worried about the same thing with latanoprost, and after 20 years, there is no indication that the protective effect of IOP lowering is lessened by a detrimental effect that increases glaucoma damage.”
Role for Rhopressa. “Rhopressa is likely to be a useful second-line treatment,” said Dr. Weinreb. “It is not quite as effective as the prostaglandins and might not be as well tolerated.” However, secondary types of glaucoma, such as steroid-induced glaucoma, may be amenable to Rhopressa because of its unique mechanism of action,” Dr. Aref noted. “Steroids increase resistance to outflow through the trabecular meshwork, but Rhopressa works to decrease it.”
A Note on Cost
When Vyzulta initially entered the market, Dr. Quigley said, his office staff was spending “a lot of time and effort” trying to get the drug for patients, as most pharmacy plans did not cover it at that point.
But coverage and reimbursement are active processes, and costs are shifting rapidly. For instance, in late June, Rhopressa was added to the preferred panel for a major plan, and the price fell to $25 per bottle for those patients.
Even as more drug plans add Vyzulta and Rhopressa, cost will be a critical issue for clinicians to discuss with their patients. In particular, the cost differential between Vyzulta and latanoprost—which has been a generic agent for about 5 years—may need to be part of the conversation, Dr. Aref noted.
___________________________
1 Food and Drug Administration. Vyzulta: Highlights of prescribing information. www.accessdata.fda.gov/drugsatfda_docs/label/2017/207795Orig1s000lbl.pdf.
2 Food and Drug Administration. Rhopressa: Highlights of prescribing information. www.accessdata.fda.gov/drugsatfda_docs/label/2017/208254lbl.pdf.
3 Weinreb RN et al. Br J Ophthalmol. 2015;99(6):738-745.
4 Weinreb RN et al. J Glaucoma. 2018;27(1):7-15.
5 Arale M et al. Adv Ther. 2015;32(11):1128-1139.
6 Serle JB et al. Am J Ophthalmol. 2018;186:116-127.
From Drought to Flood?
Here’s a sample of drugs and devices in the glaucoma pipeline.
Roclatan. In May, Aerie filed a new drug application to the FDA for Roclatan, its once-daily combination of netarsudil and latanoprost. “That could be very attractive, because the fixed-dose combination has performed significantly better than either netarsudil or latanoprost alone,” said Dr. Weinreb.
“To help with the adherence issue, we’ve wanted to see drugs combined with latanoprost for a long time,” Dr. Quigley commented. “This could be beneficial for those who need more than the prostaglandin alone.” The drug is definitely needed, added Dr. Aref. “It might be a very good first-line option for our patients. The big question is whether the combination agent is more efficacious than Vyzulta.”
Sustained-release options. In trials under highly controlled conditions, patients only use 72% of their topical glaucoma medications, said Dr. Quigley, but the estimate in the real world is closer to 50%. If clinicians could deliver a sustained-release drug in the office that lasted 6 months, he said, “it would only have to be half as effective as the eyedrop to be more effective overall. And it would also be there in a constant dose instead of in a whopping high dose followed by none at all 24 hours later.” Because sustained-release drugs do sacrifice efficacy to some degree, they may not be a first-line therapy for those without adherence issues, said Dr. Aref.
In preclinical studies, Dr. Quigley and his colleagues have experimented with subconjunctival delivery of biodegradable polymer microparticle formulations of dorzolamide. “Other research is ongoing with a variety of methods for sustained delivery,” he said.
Allergan has a biodegradable sustained-release bimatoprost implant in clinical trials that is injected into the anterior chamber, said Dr. Weinreb. An ongoing phase 3 clinical trial may answer questions about its length of efficacy and impacts on the cornea.
Microdose spray. A new technology by Eyenovia uses a variation on high-resolution inkjet printing technology that allows patients to self-administer small doses of drug to the eye, said Dr. Weinreb. This has the potential to reduce side effects and increase safety and tolerability, he said, adding that the company is planning phase 3 studies.
|
Dr. Aref is associate professor of ophthalmology and residency program director at the University of Illinois College of Medicine in Chicago. Relevant financial disclosures: None. Dr. Quigley is professor of ophthalmology at the Johns Hopkins Wilmer Eye Institute in Baltimore. Relevant financial disclosures: None. Dr. Weinreb is chairman and professor of ophthalmology at the University of California, San Diego; director of the Shiley Eye Institute in San Diego; and director of the Hamilton Glaucoma Center in La Jolla, Calif. Relevant financial disclosures: Aerie Pharmaceuticals: C; Allergan: C; Bausch + Lomb: C; Eyenovia: C.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Aref New World Medical: C.
Dr. Quigley Genentech: C; Graybug: C,O; Novartis: C; Sensimed: C.
Dr. Weinreb Aerie: C; Alcon: C; Allergan: C; Bausch + Lomb: C; Carl Zeiss Meditec: S; CenterVue: S; Eyenovia: C; Genentech: S; Heidelberg Engineering: S; Implandata: C; Konan: S; National Eye Institute: S; Neurovision: S; Novartis: C; Optos: S; Optovue: S; ORA: C; Tomey: S; Sensimed: C; Topcon Medical Systems: S; Toromedes: O; Unity: C.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|