Download PDF
Institute of Medicine workshop explores current gaps in basic science and points to promising research pathways.
On a chilly Saturday morning last November, a dedicated cadre of specialists, under the auspices of the Institute of Medicine (IOM), met in a lecture hall at the National Academy of Sciences in Washington. Determined to advance research on dry age-related macular degeneration (AMD), they took on the following objectives for the meeting:
- Examine the evidence needed to use clinical endpoints and potential biomarkers as endpoints in clinical trials, and determine how to generate that evidence.
- Discuss opportunities to improve and develop new clinical design methodologies.
- Consider scientific opportunities and challenges in speeding drug development for early intervention.
- Explore tangible next steps toward accelerating drug development.
The National Eye Institute (NEI) participated in the meeting in hope of achieving some form of agreement on the approach to studying dry AMD, said Philip J. Rosenfeld, MD, PhD, at the Bascom Palmer Eye Institute.
“We used to think of treating dry and wet AMD as separate diseases,” Dr. Rosenfeld said. “But, in reality, that’s not the case. After years of treating wet AMD and converting it back to the dry form, we are finding that these patients are going on to lose vision from the underlying dry macular degeneration.” He added, “Therapeutically, it’s not two diseases. It’s one disease, and if we can come up with a treatment for the dry, we won’t have to worry about the wet. So finding an effective treatment for dry AMD really is the Holy Grail of AMD treatment.”
Is Better Subgrouping the Answer?
Frederick L. Ferris III, MD, at the NEI, said that most researchers who are heavily involved in AMD research suspect that AMD may comprise various subgroups, all of which are currently being tossed into the same bin.
“But the new imaging and psychophysical testing techniques that are available may give us an opportunity to identify those subgroups that may be mechanistically and genetically different,” Dr. Ferris said. He added that the phenotype/genotype study group from the Arnold and Mabel Beckman Initiative for Macular Research is working toward better subgrouping of dry AMD patients, but this research is not expected to bear fruit for some time.
Dr. Ferris noted that the neovascular form of AMD is a complication of the progression of dry AMD. “Any disease that causes the breakdown of Bruch’s membrane and [retinal] pigment epithelium has the potential to encourage these abnormal choroidal vessels to start growing—and this includes trauma, infection, high myopia, or almost anything that disrupts Bruch’s membrane.” As a result, slowing the progression of dry AMD is a critical goal.
Dr. Ferris views AMD—at least the typical form of AMD—as the progression from small drusen to medium-sized drusen to large drusen, along with associated pigmentary changes, eventually leading to the development of geographic atrophy (GA). He noted, however, that very few small drusen will progress to large drusen (see “New Insights Into Drusen”). “That process takes a long time, and one of our studies showed that it takes, on average, six years to progress from large drusen to GA. The reality is that most large drusen never progress to GA. Instead, they often wind up resolving with changes in the pigment epithelium and hypopigmentation but not with this frank GA that is a major cause of vision loss.”
In the meantime, genetic associations that have been identified suggest a number of potential targets for treatment. “Industry is starting several trials that will be looking at various treatments and primarily focusing on patients who already have what I would call late AMD—or the late dry form that is GA,” Dr. Ferris said. “These trials have been initiated primarily to demonstrate that a particular treatment will slow progression of the area of GA. That is a worthwhile goal, and I hope that some of these drugs will work.” (See “A Look at the Research.”)
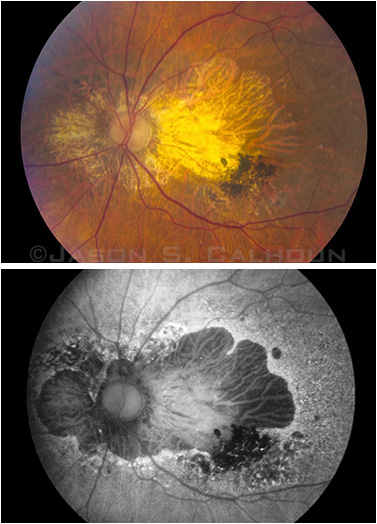 |
One patient’s GA shown by color fundus photography (top) and fundus autofluorescence imaging (bottom).
|
Identifying Genes, Searching for Clues
Despite the uptick in current research, the road forward is likely to be a bumpy one, the experts said. “The challenge is that we don’t fully understand the pathogenesis of this disease,” said Emily Y. Chew, MD, at the NEI. “There are many clues—genetics, risk factors such as smoking, and the effects of oxidative stress. Oxidative stress looks like it may be important.”
From genes to diet. A major genetics finding in 2005 suggested that the complement system might be in play, Dr. Chew noted.1-3 Since that time, a host of potentially relevant genes has been found, including complement factor H and ARMS-2. “These genes are found in chromosomes 1 and 10, and they are so important in determining a person’s risk of developing AMD,” Dr. Chew said. “But we don’t yet know exactly what these genes do or what their normal functions might be.”
Dr. Chew added that at least 20 genes have been identified and associated with AMD, but how they all connect remains elusive. Diet clearly plays a role. “Researchers in the Rotterdam Eye Study looked at genetics and diet, and they believe that even if you are dealt a very bad set of cards genetically, if you eat well and you eat the foods that you are supposed to eat, you may ‘eat away your risks,’ and that’s pretty exciting.”4
Moreover, the Blue Mountains Eye Study, the Age-Related Eye Disease Study, and NEI’s Eye Disease Case-Control Study have all suggested that diet plays an important role in macular degeneration. For instance, in the Eye Disease Case-Control Study, individuals who regularly ate green leafy vegetables, which are rich in lutein and zeaxanthin, had a reduced risk of developing late AMD. Adding fish to the diet also lowered the risk.5
Tips from twins. Johanna M. Seddon, MD, ScM, initiated the U.S. Twin Eye Study of World War II Veterans in the 1980s, using the Twin Registry sponsored by the National Academy of Sciences National Research Council. The purpose of the study was to determine the genetic and environmental influences on macular degeneration.
“We evaluated the monozygotic and dizygotic twins around the country and used our standardized protocol to evaluate them for stage of macular degeneration using our clinical age-related maculopathy grading system,” said Dr. Seddon, at Tufts University. “We found a strong genetic component of 46 to 71 percent for macular degeneration, with a higher heritability for the advanced form of the disease, and an environmental component of 19 to 37 percent. Dietary factors such as intake of omega-3 fatty acids were found to be protective, and smoking was found to have an adverse effect.” Her group and other investigators around the world have since identified numerous genes and genetic loci that are associated with both GA and neovascular AMD, explaining in part the heritability component.
Dr. Seddon added that the Dietary Ancillary study she led reported the beneficial association with dietary intake of lutein and zeaxanthin in the early 1990s, as well as the detrimental effects of increased total fat intake and consumption of dietary trans fats. The same study, along with the Progression of AMD Study, found that consumption of fish and nuts was associated with a decreased risk of progressing from the early or intermediate forms to the advanced stages of macular degeneration. The researchers also found associations with other variables related to environmental or behavioral aspects, including body mass index (BMI), waist-to-hip ratio, and risk reduction through regular physical activity. They also looked at cardiovascular risk profiles among individuals with AMD and at serum levels of C-reactive protein, a marker for systemic inflammation that also increases the risk of AMD progression from early and intermediate stages to the advanced form of the disease.
Advice for patients. Practical takeaways from Dr. Seddon’s research are as follows: Don’t smoke; follow a healthful diet rich in dark green leafy vegetables and low in fat; eat fish a few times a week; maintain a normal weight and waist size; exercise regularly; and control blood pressure and cholesterol. She added that anyone who has signs of intermediate-level macular degeneration in both eyes or advanced macular degeneration in one eye should take dietary supplements that contain lutein, zeaxanthin, vitamin C, vitamin E, and zinc.
New Insights Into Drusen
Dr. Ferris’ group at the NEI is using data from the Age-Related Eye Disease Study to better understand the process that ultimately leads to GA. “We find that just about everyone will develop small drusen as part of the aging process,” Dr. Ferris said. “In fact, one of the first things the Beckman phenotype/genotype group did was to write a paper on the classification of AMD1 in which they said that they don’t like calling small drusen ‘AMD.’ Instead, they suggested that these be called ‘normal aging changes.’”
Dr. Ferris added that current data suggest that small drusen are not a risk factor for progressing to large drusen. “We have shown that it’s not the little drusen that are a problem, and we don’t want to worry patients needlessly by telling them that they have early AMD. But when you get to the medium-sized drusen, that’s when you say, ‘This patient is potentially on her way to GA.’ We base this on a 10-year follow-up of patients, and among those with small drusen, very few progress to large drusen. But patients who have medium-sized drusen in both eyes have a 50 percent chance of having large drusen within five years.
“This is a real distinction between little drusen and medium-sized drusen,” Dr. Ferris continued. “As a result, we believe that it’s the medium-sized drusen that are the hallmark of the earliest stages of AMD. That’s not to say that these little drusen aren’t important. They may be ‘necessary but not sufficient’ and may get the process started, especially among patients who have genetic abnormalities like complement factor H or ARMS-2. A lot of us believe that genetic abnormalities at that early stage may trigger this, perhaps by an improper immune response to the presence of this new drusen material in the extracellular space.”
Dr. Ferris added that researchers would like to examine these patients with medium-sized drusen more carefully. “We have designed a five-year study to follow these patients and measure how these drusen accumulate and whether there are high-risk patients who accumulate drusen more quickly or who go on to large drusen more quickly,” he said. “Then we would like to look for genetic changes among those who progress or who progress more slowly in the medium-sized group. We also would like to use optical coherence tomography to determine whether we can identify patients who are progressing based on drusen volume.”
___________________________
1 Ferris FL et al. Ophthalmology. 2013; 120(4):844-851.
|
Seeking Solutions
A number of potential treatment strategies for dry AMD are under investigation. These include prevention of oxidative damage, improvement of choroidal blood flow, neuroprotection, modulation of the visual cycle, modulation of the immune system, and stem cell transplantation (see “A Look at the Research”).
Leading candidate. Complement inhibition is also generating interest and is the first treatment strategy to reach phase 3 trials. Dr. Ferris said that the large phase 2 MAHALO trial, which tested lampalizumab and appeared to show some degree of efficacy, has now led to two large phase 3 studies—CHROMA and SPECTRI.
Still early days. However, Daniel F. Martin, MD, urged caution, as most therapies are in the early stages of development. With regard to the IOM workshop, he noted, “What this conference really focused on was: 1) Is there a clearly identified therapeutic target? 2) What would be the optimal clinical trial endpoint? and 3) How can we conduct these trials more efficiently?”
Dr. Martin, who is at the Cole Eye Institute, pointed out that GA is a slow process, and this—combined with uncertainty about the drug target—means that trials will take a long time to complete and will require many patients. “This makes the trials very expensive, and that is the real challenge for the pharmaceutical industry. Exploring ways to enrich patient selection might decrease the time and expense, and this was a focus of the meeting.”
A Look at the Research
A number of therapies for geographic atrophy are being investigated in clinical trials. Here’s a brief overview; more information regarding trial status is available at www.clinicaltrials.gov.
| Drug |
Mechanism of Action |
Mode of Delivery |
| Lampalizumab |
Inhibits complement factor D |
Intravitreal injection |
| LFG316 |
Human antibody that targets complement component 5 |
Intravitreal injection |
| Oracea (doxycycline) |
Suppresess inflammation; tetracycline derivative |
Oral |
| Iluvien (fluocinolone acetonide) |
Suppresses inflammation |
Intravitreal implant |
| Brimonidine tartrate |
Neuroprotective; an alpha-2 adrenergic receptor agonist |
Intravitreal implant |
| GSK933776 |
Neuroprotective; humanized monoclonal antibody directed against the N-terminal sequence of amyloid beta |
Intravenous |
| MC-1101 |
Modulates choroidal blood flow |
Topical |
| Emixustat hydrochloride |
Visual cycle modulator; inhibits activity of RPE65 |
Oral |
| MA09-hRPE cells |
Stem cell therapy; involves transplantation of hESC-derived RPE cells |
Stem cell implant |
| HuCNS-SC cells |
Stem cell therapy; involves transplantation of HuCNS-SC cells into subretinal space |
Stem cell implant |
NOTE: hESC = human embryonic stem cell; HuCNS-SC = human central nervous system stem cell; RPE = retinal pigment epithelium. SOURCE: Philip J. Rosenfeld, MD, PhD.
|
Can Alzheimer Disease Lead the Way?
As he listened to a presentation on Alzheimer disease at the IOM’s workshop, Dr. Martin was struck by the similar challenges posed by the two diseases.
Surprising similarities. In a session on how to enrich clinical trials and find ways to conduct clinical trials that are shorter and less expensive, William Z. Potter, MD, PhD, was the first speaker—and, in many ways, the most interesting, said Dr. Martin. “There are a lot of similarities between dry AMD and Alzheimer disease. Right now, we are not even sure what the right target is in AMD. A few have been proposed, but according to Bill Potter, when you look back at Alzheimer disease, there were more than 150 different targets, and only one was ever validated.”
Dr. Potter, a senior advisor to the National Institute of Mental Health, recalled those early years in Alzheimer disease research. “You had a lot of efforts aimed at targets that might not have been the right targets,” he said at the meeting. “Also in Alzheimer disease, many of the targets you were aiming at were pretty late in the disease.” What it ultimately took was the formation of a network that focused on discovery (although researchers did conduct some trials later). But there was a concerted effort within this network to develop rational targets for potential treatment.
Need for coordinated effort. Listening to Dr. Potter’s description of the path taken by Alzheimer disease researchers, Dr. Martin realized the importance of creating a similar effort. “There is a tremendous need for a network that would be devoted to identifying AMD targets and conducting testing,” Dr. Martin said. He imagines potentially conducting phase 2 and possibly even phase 3 clinical trials while facilitating the understanding of appropriate targets and working in collaboration with industry or with whoever has a good idea for early trials for GA.
Without such coordination, Dr. Martin added, researchers will continue to be saddled with a disorganized, somewhat disjointed effort in which even the targets are not well defined. “As a result, the likelihood that our efforts are going to lead to an effective treatment is low. But we are going to learn a tremendous amount from the lampalizumab trials. Maybe we’ll get lucky, and lampalizumab will end up being highly effective. If it doesn’t work, we will have learned a lot, but it could take many more years before we have the right target”—and, again, that was the Alzheimer’s story.
Hopes and Challenges
Despite the long road ahead, Dr. Rosenfeld is optimistic about the future of AMD research and treatment. “When I started on faculty more than 18 years ago, wet AMD resulted in an almost guaranteed loss of vision. We would do what we could, but we knew that over several weeks to months, the patient was going to lose central vision. Once in a while, we hit a home run, but most of the time we felt like we were hitting singles and getting thrown out trying to steal second. But now, wet AMD really isn’t the problem. We have been able to stop the worst of the rapid, devastating vision loss, and I believe that we can tackle the issues surrounding dry AMD.”
Currently, ophthalmologists are focusing on slowing GA in dry AMD, but that may be too late in the disease process, Dr. Rosenfeld added. “I think we have to start treating earlier. The IOM meeting was valuable in achieving agreement on how we might target the disease at an earlier stage so that even more vision can be preserved.”
___________________________
1 Klein RJ et al. Science. 2005;308(5720):385-389.
2 Edwards AO et al. Science. 2005;308(5720):421-424.
3 Haines JL et al. Science. 2005;308(5720):419-421.
4 Ho L et al. Arch Ophthalmol. 2011;129(6):758-766.
5 SanGiovanni P et al. Arch Ophthalmol. 2007;125(5):671-679.
Meet the Experts
EMILY Y. CHEW, MD Medical retina specialist and deputy director of the Division of Epidemiology and Clinical Applications at the NEI. Participated in IOM workshop. Financial disclosure: None.
FREDERICK L. FERRIS III, MD Director of the Division of Epidemiology and Clinical Applications at the NEI. Served as facilitator for the IOM workshop. Financial disclosure: A U.S. patent entitled “Nutritional Supplement to Treat Macular Degeneration” (Patent No. 6,660,297) was issued on Dec. 9, 2003; Dr. Ferris is one of the inventors. The patent is owned by Bausch and Lomb. Dr. Ferris has assigned his interest in the patent to the U.S. Government and receives government compensation.
DANIEL F. MARTIN, MD Chairman of Cole Eye Institute in Cleveland. Participated in IOM workshop. Financial disclosure: None.
<pPHILIP J. ROSENFELD, MD, PHD Professor of ophthalmology at Bascom Palmer Eye Institute in Miami. Participated in IOM workshop. Financial disclosure: Has served as a consultant to Acucela and received grant support from Acucela, Advanced Cell Technology, Genentech, and GlaxoSmithKline.
JOHANNA M. SEDDON, MD, SCM Professor of ophthalmology at Tufts University School of Medicine and director of the Ophthalmic Epidemiology and Genetics Service at Tufts Medical Center in Boston. Participated in IOM workshop. Financial disclosure: Has received grant support from Genentech.
|