Download PDF
Neuroprotectors. Visual cycle modulators. Antioxidants. Complement inhibitors. Anti-inflammatory agents. All these are under investigation as therapies to stop dry AMD. While there is no question that it will take years before a proven treatment is available, the ongoing research is expected to yield a rich harvest of data.
In addition to these investigational approaches, some novel avenues are opening to researchers. Four experts discuss the state of the research—and some critical questions that remain unanswered.
Drugs Under Investigation
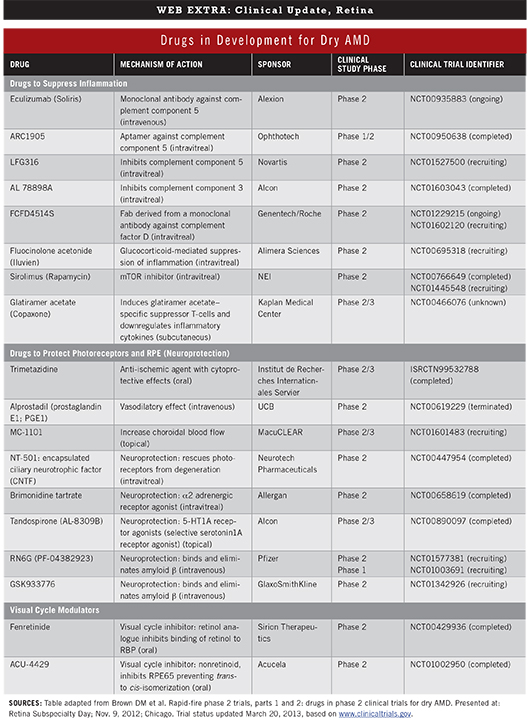
Because the pathophysiology of dry AMD is not yet fully understood, researchers are pursuing several possible therapeutic mechanisms of action. Four major mechanisms comprise the majority of clinical trials.1 (For a list of drugs and trials, see "Drugs in Development for Dry AMD.")
Inflammation suppression. A number of drugs are under investigation for their role in suppressing inflammation in AMD. In particular, “A lot of people are very optimistic about the complement pathway because we know that complement factors and mutations in the complement pathway increase your risk of developing macular degeneration,” said Peter K. Kaiser, MD, chairman of ophthalmology research at the Cleveland Clinic’s Cole Eye Institute.
For instance, various complement components have been identified in drusen, within Bruch’s membrane, and in the anterior choroid; in addition, patients with complement-mediated renal diseases have been found to have deposits resembling drusen. Nevertheless, many questions remain about the role of the complement cascade in dry AMD (see “Known Unknowns and the Complement Cascade”).
Neuroprotection. In an attempt to slow or prevent the development of drusen, pigment abnormalities, and geographic atrophy, several companies are testing drugs to protect photoreceptors and the retinal pigment epithelium (RPE). Unfortunately, at least three of four recent neuroprotection studies have failed, and results of the fourth are pending, said Dr. Kaiser. “Neuroprotection, even in glaucoma, hasn’t been shown to work. In macular degeneration, all of the studies to date have been disappointing.”
One novel avenue of neuroprotection under investigation involves anti-amyloid therapy, based on Alzheimer disease research, as amyloid is present in both diseases.1
RPE regeneration or rescue. Stem cells are being used to regenerate RPE or rescue photoreceptor loss in geographic atrophy. Two companies are currently in phase 1 trials with this approach, Dr. Kaiser said.2
Visual cycle modulation. Accumulation of the toxic by-product lipofuscin within the RPE is a part of the visual cycle. It’s also a prominent pathologic feature of dry AMD. Visual cycle modulators are intended to reduce the accumulation of these toxins by slowing the visual cycle.
“Conceptually, visual cycle modulators currently hold the most promise,” said Dr. Kaiser. The studies are furthest along, at least in the form of fenretinide. The drug has shown some benefit in a phase 2b trial, although side effects include poor night vision and poor dark adaptation. However, patients can overcome the adaptation problem in a few weeks, said Pravin U. Dugel, MD, managing partner at Retinal Consultants of Arizona in Phoenix and clinical associate professor of ophthalmology at the Doheny Eye Institute in Los Angeles.
Known Unknowns and the Complement Cascade
Over a decade’s worth of research has implicated the role of the complement cascade in the development of AMD. Still, a number of questions remain.
Questioning the process. “We use the catchall term of ‘complement modulation,’ yet we can’t even say what pathway in the complement cascade is involved,” said Dr. Kaiser. Among the questions needing resolution: Most complement is produced outside the eye, so should we treat with a systemic or local blocker? What triggers the cascade? Is a pathogen, such as a foreign body or microbe, involved? And is it a lifelong problem, or do patients hit a tipping point that spurs AMD development?
Finally, we don’t know the risks of inhibition, said Dr. Kaiser. “The complement cascade is a vital part of our immune system. If you block it, you’re blocking a key component of the immune system. That might be a problem.”
Dr. Kaiser is intrigued, however, by some of the more specific alternative pathway complement inhibitors, in particular those that replace abnormal complement factor H with a normal version. “That’s a very interesting and creative way of attacking the problem, which shouldn’t cause issues outside the alternative pathway,” he said.
Questioning the role of genetics. Companies started doing complement inhibitor trials on the basis of findings in the mid-2000s implicating complement factor H as a genetic risk factor for geographic atrophy, said Dr. Ambati. “But there is not a synonymous relationship between statistical genetics and disease mechanism.”
“The genetic association with complement factor H is huge,” but it hasn’t translated into a treatment, said Dr. Curcio. She added that a straightforward way to study the association is to knock the gene out. “Take out that gene, what happens? What they found was the Bruch’s membrane got thinner instead of thick and photoreceptors degenerated. They didn’t see drusen.”
|
Running Into Roadblocks
There’s no question that dry AMD “is not an easy disease to study. It’s not an easy pathophysiologic process,” said Dr. Dugel. And the disease progresses slowly; it is a “very long disease,” said Dr. Kaiser. “A lot of these drugs may be having an effect. But in a small or short study we don’t see that.”
Moreover, we are still missing pieces of the puzzle of AMD biology, said Christine A. Curcio, PhD, professor of ophthalmology at the University of Alabama in Birmingham. “But we do know a lot about some, like choroidal neovascularization, and we now have a big clue on drusen. And we have testable hypotheses about how the eye works.” Her hypothesis involves what she calls an “oil spill” in Bruch’s membrane (discussed below).
Given the challenges, it’s easy to become frustrated with the pace of dry AMD research, as each step forward seems to bring corresponding steps backward. For instance, Dr. Curcio cited new research that calls into question the role of lipofuscin-related cell death as well as the rationale of treatments targeting lipofuscin.3
(click to expand)
Charting New Courses
Despite research roadblocks, investigators continue to forge ahead in novel areas of research.
Aging and lipoproteins. Because advancing age is the biggest risk factor for AMD, Dr. Curcio posed the question: What happens during aging that is a clue to this disease? She found an answer in cardiovascular disease.
Specifically, she saw a parallel between the process of drusen formation in Bruch’s membrane (and between the RPE and the membrane) and that of lipid deposition in cardiovascular vessel walls.
Then she homed in on an age-related accumulation of cholesterol in Bruch’s membrane, eventually discovering that the RPE produced cholesterol-rich lipoprotein particles with apolipoprotein B and E.4,5 “For 40 years, people said what ends up in Bruch’s came from the photoreceptors. But when we analyzed the fatty acids, they do not resemble the photoreceptors’ product.” Instead, she said, they resemble dietary components.
“Our model is that the RPE takes up cholesterol-rich lipoprotein from plasma to meet the metabolic requirements of the retina. The RPE strips out what it needs to send to photoreceptors and gets rid of excess through a recycling back to Bruch’s membrane. As years go by, Bruch’s membrane thickens, and it gets cross-linked and harder to clear. Lipoproteins eventually form a layer on the surface,” Dr. Curcio said, which she calls the “oil spill.”
Over time, it becomes peroxidized, and then expands volumetrically into drusen. The lipids become toxic and proinflammatory. Peroxidized lipids kill cells and block nutrition from getting to the RPE and photoreceptors.
“Drusen formation follows a lifetime of building up a tremendous lipid-rich barrier in Bruch’s membrane,” she said. “Think of drusen as tar balls in an oil spill.”
Dr. Curcio has begun to create in vitro “oil spills,” which may validate one aspect of her model. “That is, a cell can live in a dish and make drusen components without having photoreceptors there,” she said. “This suggests that the pathway’s driving force is lipoproteins derived from diet and not processed photoreceptor outer segments, although photoreceptors undoubtedly contribute to the final composition.”
She likened one possible treatment approach to the “top kill” maneuvers used to plug gushing oil wells. “The equivalent in the eye would be to modify the output of lipoproteins from the RPE,” she said. “If you could turn that down, maybe less would accumulate.”
If Dr. Curcio is correct and the accumulation of lipids in Bruch’s is similar to atherosclerotic cardiovascular disease, ophthalmic drug researchers may be able to benefit from the work done in cardiovascular drug development. She said she hopes to see some oil spill–based treatments within eight years, “if small-molecule drugs from cardiovascular disease are tested concurrently with relevant laboratory studies, and we begin today.”
New immune system component. Based on evidence for inflammation in AMD pathogenesis (drusen contain a number of proteins associated with inflammation), researchers have hypothesized a role for a component of the immune system known as inflammasome.
One researcher investigating this avenue is Jayakrishna Ambati, MD, professor of physiology and professor and vice chairman of ophthalmology and visual sciences at the University of Kentucky in Lexington.
In 2011, Dr. Ambati’s team reported that eyes in people with geographic atrophy have a deficiency of the DICER1 enzyme in the retina. DICER1 maintains visual health by degrading the RNA molecule, Alu RNA.6 Insufficient levels of this enzyme allow toxic accumulation of Alu RNA. The research team also showed that Alu RNA accumulation activates an immune complex known as NLRP3 inflammasome. This triggers a process that causes RPE cells to die.7
In laboratory models, Dr. Ambati’s team demonstrated efficacy of two therapies aimed at preventing geographic atrophy. One involves increasing DICER1 levels in the RPE by overexpressing the enzyme. The other binds to and breaks down Alu RNA.
Other labs have reported that eyes in people with geographic atrophy display activation of the inflammasomes and cell death, Dr. Ambati said. “So there’s a convergence in the field from multiple different labs that inflammasome activation is very important in geographic atrophy.”
|
"Oil Spill" Hypothesis
|
 |
|
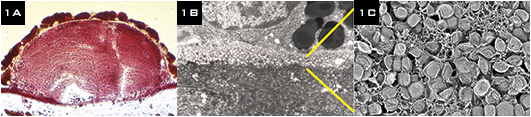
(1A) Oil red O staining reveals abundant lipid in drusen. (1B A 3 µm-thick layer on Bruch’s membrane is packed with solid lipoprotein particles, (1C) shown at higher magnification, using a lipid-preserving quick-freeze deep-etch technique. Credits: (1A) G. Malek and C.A. Curcio (1B, 1C) C.A. Curcio et al. Br J Ophthalmol. 2011;95:1638–1645.
|
Hope on the Horizon
Overall, we shouldn’t be discouraged by any research failures, said Dr. Dugel, who is most intrigued by the hypothesis of visual cycle moderation. “We understand more about the disease process than we did five years ago. I’m very encouraged that we’ll have some kind of treatment within five years,” he said.
“It’s too early to say which technique is going to work,” Dr. Kaiser acknowledged. “But at least we’re moving ahead. When I entered practice, there was no dry AMD therapy at all. Now we have at least AREDS vitamin supplementation.”
For now, he said, “It’s important for doctors to give patients some hope. We’re working on this. We’re not sitting around and saying, ‘I’m sorry.’ We are working on trying to get patients a treatment for this pervasive problem.”
___________________________
1 Brown DM et al. Rapid-fire Phase 2 Trials, Parts I and 2: Drugs in Phase 2 Clinical Trials for Dry AMD. Presented at Retina Subspecialty Day; Nov. 9-10, 2012; Chicago.
2 Du H et al. Semin Ophthalmol. 2011;26(3):216-224.
3 Rudolf M et al. Ophthalmology. 2013 Jan 25 [Epub ahead of print].
4 Curcio CA. Br J Ophthalmol. 2011;95(12):1638-1645.
5 Johnson LV et al. Proc Natl Acad Sci U S A. 2011;108(45):18277-18282.
6 Kaneko H et al. Nature. 2011;471(7338):325-330.
7 Tarallo V et al. Cell. 2012;149(4):847-859.
___________________________
Dr. Ambati is a cofounder of Iveena Pharmaceuticals. Dr. Curcio is a consultant for Roche; her research is funded by NEI and supported by local foundations in Alabama. Dr. Dugel is a consultant for Acucela, Genentech, and Regeneron. Dr. Kaiser is a consultant for Alcon, Bausch + Lomb, Bayer, Genentech, Novartis, Ophthotech, Oraya, and Regeneron.