Download PDF
A massive new study of retinal degeneration has concluded that, long before retinitis pigmentosa (RP) has progressed to complete blindness, deterioration of rod photoreceptors leads to rewiring events within the retina—a process that previously was thought to commence much later in the disease.1
“RP in many ways is a black box mystery,” said lead author Rebecca L. Pfeiffer, PhD, at the University of Utah’s Moran Eye Center in Salt Lake City. “We know these changes in retinal circuitry occur as photoreceptors die, leading to blindness. But our research points to much smaller-scale processes before that, which could be important to developing therapeutics in the future,” she said.
The scientists’ painstaking examination of thousands of transmission electron microscope images revealed that rod photoreceptor postsynaptic target cells begin making new neural/synaptic connections to cone photoreceptors prior to complete loss of rod photoreceptors. These results, and evidence of other kinds of neural rewiring, were found by an ambitious project to construct a “pathoconnectome” for RP retinas.1
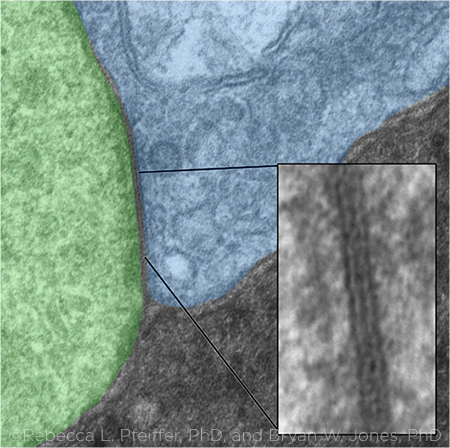 |
GAP JUNCTION. This image shows a gap junction in a transmission electron micrograph between an Aii glycinergic amacrine cell (green) and a rod bipolar cell (blue). The gap junction between them (inset) is shown magnified to 25,000×.
|
3-D visualization. The researchers detected the structural changes in retinal neural networks by stacking serial images from transmission electron microscopy into a 3-D visualization of a retinal segment. The visualization showed not only the cells present but also the synaptic connections between them (a “connectome”), Dr. Pfeiffer said. (Molecular probes helped the researchers identify phenotypes of the visualized cells.)
This study builds upon earlier research: In 2011, the researchers assembled the first-ever retinal connectome, built with images from a normal rabbit’s retina.2 In the current study, they followed up by building a pathoconnectome that shows neural network alterations in a rabbit model of retinal degeneration demonstrating a form of early, cone-sparing RP.
At this stage, some rods had already died, but many others remained morphologically intact. “The remaining rod photoreceptors, along with the inner retinal cell population, allowed for connectomics-based evaluation of early stage RP rewiring, corresponding to a clinical point where human patients would still have vision,” the researchers wrote. However, the patients “would likely be suffering from scotopic deficits as well as adaptation deficits.”1
Mixed signaling. Examination of the rod synaptic network showed that rod bipolar cells, which normally synapse with rod photoreceptors, react to the partial loss of the rods by sprouting neurites to connect with cone photoreceptors while maintaining contact with the remaining rods, Dr. Pfeiffer said. Simultaneously, rod bipolar cells form gap junctions with other cell types, including Aii amacrine cells (a common synaptic partner in the inner retina), although the rod bipolar cells never make gap junctions in the healthy retina, she said.
“While they’re maintaining some input from the rod photoreceptors that are still in place, they’re also getting input from the cone photoreceptors as well. There is mixed signaling going on,” Dr. Pfeiffer said. “You have a bleeding of signals between the day and night vision pathways that doesn’t exist in a healthy retina.”
How the pathoconnectome was built. The studies are being performed in an NIH-funded connectomics research laboratory headed by Bryan William Jones, PhD, at the University of Utah’s Brain Institute and Moran Eye Center. “We are taking apart and reconstructing the retina at nanometer scale—and exploring how those connections are broken in retinal disease,” he said.
The lab constructed the pathoconnectome by studying 946 tissue sections, just 70 nm thick, obtained with two transmission electron microscopes.
What’s next? The researchers have already gathered images and data for two other retinal pathoconnectomes, reflecting later stages of RP, but these must be further studied before publication, Dr. Jones said.
The analyses likely will be helpful for understanding not only retinal neurodegeneration but also other degenerative conditions, such as Parkinson and Alzheimer diseases, he said.
—Linda Roach
___________________________
1 Pfeiffer RL et al. Exp Eye Res. 2020;199:108196.
2 Anderson JR et al. Mol Vis. 2011;17:355-379.
___________________________
Relevant financial disclosures—Dr. Pfeiffer: None; Dr. Jones: University of Utah Research Foundation: P.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Jeng EyeGate Pharmaceuticals: O; GlaxoSmithKline: C; Kedrion: C; Merck: C.
Dr. Jones University of Utah Research Foundation: P. Callahan SP et al, inventors; University of Utah, assignee. Microscopy visualization. US patent 20140126801 A1. May 8, 2014. Jones BW et al., inventors; University of Utah, assignee. Disease diagnosis and treatment using computational molecular phenotyping. US patent 20130023436 A1. Jan. 11, 2011.
Dr. Pfeiffer None.
Dr. Repka NEI: S.
Dr. Seibold Allergan: C; New World Medical: C.
Dr. Wang Beijing University-CMU: S; Ministry of Science and Technology of the People’s Republic of China: S; National Natural Science Foundation of China: S; Sanming Project of Medicine in Shenzhen: S.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review