By Lori Baker-Schena, MBA, EdD, Contributing Writer, interviewing Amar Agarwal, MS, FRCS, FRCOphth, Alan N. Carlson, MD, Kathryn A. Colby, MD, PhD, Ahad Mahootchi, MD, and Donald T.H. Tan, MD, FRCS, FRCOphth
Download PDF
Advances in endothelial keratoplasty are giving patients with corneal endothelial dysfunction ever-more novel treatment options even though Descemet stripping automated endothelial keratoplasty (DSAEK) and Descemet membrane endothelial keratoplasty (DMEK) are the mainstays. “The field of endothelial keratoplasty is a dynamic one; it has been revolutionized in the last 20 years, and we are continuing to refine our surgical approaches,” said Kathryn A. Colby, MD, PhD, at the University of Chicago.
Some might say that this trend of innovation is attributable in part to the fact that DSAEK and DMEK have their drawbacks. Amar Agarwal, MS, FRCS, FRCOphth, at Dr. Agarwal’s Eye Hospital and Eye Research Centre in Chennai, India, noted, “In DSAEK, the donor tissue is about 80 to 150 microns. So, effectively, a 500-μm cornea will become a 600- to 650-μm cornea [after surgery], and the endothelium has to pump more.
“In DMEK, the donor tissue is only 15 μm, which is good,” he said. However, for this procedure, he noted that only grafts from older donors can be used. This is because in donors younger than age 40-50, the endothelium adheres so strongly to the stroma that they cannot be separated.
Enter pre-Descemet endothelial keratoplasty (PDEK), an emerging procedure intended to address both challenges. In another corner of the field, Donald T.H. Tan, MD, at the Singapore National Eye Centre, is developing hybrid DMEK (H-DMEK, see sidebar), another technique designed to combine the best of both DSAEK and DMEK.
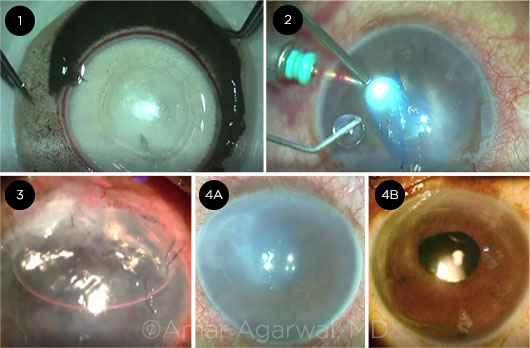 |
STEPS. Stills from this PDEK video show (1) creation of the Type 1 Big Bubble to harvest the donor graft, (2) graft injection into recipient eye, (3) the eye on postop day 1, and (4A,4B) the preop eye compared with the eye at 3 months postop.
|
PDEK
Dr. Agarwal performed the first PDEK procedure in India in September 2013 and described the technique in March 2014 in the British Journal of Ophthalmology.1 Candidates include patients with endothelial decompensation such as Fuchs dystrophy and pseudophakic or aphakic bullous keratopathy, he said.
The procedure involves transplanting only 25 μm of tissue: the pre-Descemet layer, the Descemet membrane, and the endothelium. “The pre-Descemet layer gives a splinting effect to the graft and makes it easy to manipulate,” said Dr. Agarwal. Several benefits of PDEK are directly attributable to the graft’s relative stiffness, he said.
Bigger donor pool. Unlike DMEK, which relies on grafts from donors aged 40 years and older, PDEK has no donor age restriction, said Dr. Agarwal. “The youngest we have used is a 9-month-old donor.2 This gives us the big advantage of healthier grafts with a better endothelial cell count.”
Easier manipulation. As with DSAEK, PDEK grafts can be manipulated more easily than DMEK grafts, Dr. Agarwal said. Alan N. Carlson, MD, at Duke Eye Institute, in Durham, North Carolina, agreed, noting that DMEK can be unpredictable. “You could have the perfect procedure, and the graft tissue itself would end up scrolling or detaching. With PDEK, I feel I have better control of the tissue because of the added Dua (pre-Descemet) layer.”
Advantage for complex patients. Ahad Mahootchi, MD, in private practice in Zephyrhills, Florida, noted, “To get the DMEK graft to unscroll, surgeons intentionally shallow the anterior chamber. But in complicated situations, such as in patients with vitrectomized eyes or those with prior Nd:YAG posterior capsulotomy in whom the anterior chamber can be difficult or impossible to shallow, it may be a challenge to unroll the DMEK graft. PDEK grafts are ideal for these complex patients,” he said.
Dr. Colby added that in some patients, you may not want to do a DSAEK, but you can’t do a DMEK. She said that she sees a case for PDEK or H-DMEK in patients with aphakia, iris defect, or a vitrectomized eye—situations in which the anterior chamber might not sufficiently shallow for successful unscrolling of a DMEK graft, or where DMEK tissue may be at risk of falling through to the posterior segment.
Lower detachment risk? Based on what he’s seen in his practice, Dr. Agarwal said that the 10-μm pre-Descemet layer lowers the risk of detachment compared to DMEK. And in correspondence to the Journal of Cataract and Refractive Surgery, Dr. Agarwal and colleagues reported outcomes in 12 PDEK patients. Eight grafts adhered successfully, and there were 4 detachments, 3 of which did not require intervention. None detached completely.3
Good visual outcomes. Of the cases he has done so far, Dr. Agarwal said that postoperative edema improves, and vision stabilizes in PDEK patients as rapidly as it does in DMEK patients. In the same 12-patient study, corrected distance visual acuity (CDVA) at 90 days was between 20/35 and 2/90 for all but 2 patients whose CDVA was 20/200, and at 1 month, 1 eye had minimal interface haze.3
Dr. Agarwal added that PDEK may have a role for badly scarred corneas. In a prospective, interventional study, Dr. Agarwal’s group reported on 4 patients with chronic pseudophakic bullous keratopathy who underwent PDEK or coupled with epithelial debridement. The 4 gained between 1-5 lines.4 (Two additional patients underwent DMEK with debridement, each gaining 2 lines.)
Drawbacks. Because most U.S. eye banks don’t prepare PDEK tissue, the surgeon may have to prepare the donor graft, and this can be tricky (see “Preparing the PDEK Graft”). That said, a few local eye banks do prepare this tissue (see “Eye Banks’ Key Role”).
Hybrid DMEK
The basic idea behind hybrid DMEK (H-DMEK) is to make it more like DSAEK, said Donald Tan, MD, FRCS, FRCOphth, in Singapore. “This is because there is greater reproducibility with DSAEK and surgeons are more comfortable with it, whereas DMEK requires much skill and can be more stressful for the surgeon.”
What is hybrid DMEK? H-DMEK uses a standard DSAEK approach with a few differences. During donor graft preparation, the Descemet membrane and endothelium is separated from the pre-cut DSAEK tissue, the DMEK graft is laid back loosely over the stroma, and the whole complex is coiled into an EndoGlide Ultrathin DSAEK inserter (Network Medical Products). Once the EndoGlide is inserted inside the anterior chamber, the surgeon uses intraocular forceps to pull only the DMEK tissue from the inserter, leaving the stroma in the cartridge, which is then removed. The DMEK tissue is always right-side up, and the forceps provides enhanced surgeon control over the DMEK tissue, which tends to naturally uncoil of its own accord in the anterior chamber.
Results. Dr. Tan just completed a clinical series of about 90 H-DMEK cases in both standard and more challenging cases, which he plans to submit for publication. Of the cases, over 40 were in eyes that ordinarily would be unsuitable for DMEK. These were performed in the latter half of the series, once the technique had been refined. These complex cases included aphakia, aniridia, vitrectomized eyes, eyes with tube shunts, and previous failed penetrating keratoplasties or DSAEK.
Despite the difficulty of these cases, the series had a relatively low primary graft failure rate of 2.2% and a rebubbling rate of 8.8%. There were 2 documented cases of endothelial rejection, which were subsequently reversed with topical steroids.
He reports that in his own practice, his H-DMEK endothelial cell loss rates are about 24% now that he has become very proficient with the procedure. When he first started, his cell loss rates were 37%, and he has brought these rates down steadily with practice.
Benefits. Dr. Tan said that H-DMEK gives enhanced surgical control of the graft tissue and also control of the anterior chamber with the EndoGlide (coupled with an anterior chamber maintainer). This allows for a closed chamber procedure, which minimizes fluctuation of anterior chamber depth. Both are important to a successful procedure and allow surgeons to tackle these more challenging cases, he noted.
He added that because the tissue is always held right-side up with the forceps, it is generally not possible to have an inadvertent “upside-down” graft. Further, this means that additional donor preparation steps such as the placement of an “S” stamp mark, to signify the exact orientation of the tissue, is not required.
Considerations. Because H-DMEK utilizes the EndoGlide DSAEK surgical approach, Dr. Tan recommended that surgeons have sufficient experience with this form of inserter and with using this “pull-through” forceps technique before attempting or adapting the H-DMEK technique to their practice.
Of note, Dr Tan has developed a new DMEK EndoGlide inserter specifically for DMEK surgery, which may further simplify the H-DMEK procedure. It is currently undergoing clinical trials.
|
The PDEK Procedure
Dr. Agarwal describe the procedure as follows.
Insertion. The PDEK graft is inserted into the anterior chamber through a microincision lens injector using generally the same technique as that used in DMEK. The graft is unrolled and floated up against overlying stroma by injecting air.
Attachment. Under pressurized air infusion, the reverse Sinskey hook is used to engage the PDEK graft in the periphery, and the graft is centered into position within the descemetorhexis. “Under continuous air support, any graft edges that are folded inward are unfolded using a reverse Sinskey hook or a thin, blunt rod introduced between the graft and the host stroma through the sideport,” Dr. Agarwal explained. “Wrinkles and creases in the graft are also stretched out with the reverse Sinskey hook.”
Case Study
Dr. Mahootchi’s first PDEK patient was a 60-year-old Canadian woman who wintered in Tampa and had already undergone 3 failed corneal transplants, as well as a vitrectomy and cataract surgery followed by a Nd:YAG capsulotomy—all in her right eye. Her best-corrected VA was hand motions in the right eye and 20/20 in the left. She was taking high-dose topical steroids to prevent corneal transplant rejection, which resulted in uncontrolled glaucoma despite using 3 medications. The inflammation and steroid use had also caused a recurrent ulcer.
“I chose to clear up the cornea with a PDEK graft and performed transscleral cyclophotocoagulation in an attempt to reduce the need for both steroids and glaucoma medication,” he said.
“My first thought in this approach was to lessen her medication load, and within a few weeks she was able to stop taking both the steroids and topical glaucoma therapy,” Dr. Mahootchi said. “And with the new endothelium, she has been able to improve her vision to 20/400 in the right eye after a year. We dramatically improved the quality of her life.”
|
Preparing the PDEK Graft
According to Dr. Agarwal, one of the distinguishing features of PDEK is the preparation of the graft, which is accomplished by creating a Type 1 Big Bubble (BB).
Type 1 BB. In this approach, injection of air separates the pre-Descemet layer (Dua layer)/Descemet membrane/endothelium complex from the residual stromal bed. The graft is approximately 25 μm thick and 7.5 to 8 mm in diameter. Research has shown that the Dua layer confers additional strength to the recipient cornea.5
Type 2 BB. This contrasts with the Type 2 BB in which the Descemet membrane is separated from the posterior surface of the Dua layer by the air bubble that extends to the corneal periphery. This type of bubble yields a larger graft (approximately 15 μm thick and 10 mm in diameter) with a thinner wall that is more susceptible to tears and bursting.5
“The Type 1 BB is created using a 30-gauge needle, bevel up, connected to a 5-mL syringe. This type of BB never extends to the extreme periphery due to adhesions between the pre-Descemet layer and the residual stroma,” Dr. Agarwal explained. “If a Type 2 BB graft is accidentally harvested, we need to convert the graft and surgical approach to DMEK.”
Creating the Type 1 BB to harvest the PDEK graft is essential but can be tricky, time-consuming, and potentially expensive. Possible complications in preparing the graft include Descemet membrane microperforations and a burst bubble, said Dr. Agarwal.
Dr. Colby said that she would be concerned about risk of tissue damage with PDEK. “The surgeries that we have, DSAEK and DMEK, are really pretty good, and right now, the eye banks are preparing the tissue for us.” Indeed, said Dr. Carlson, eye bank preparation of PDEK tissue for surgeons is a “big hurdle” that PDEK faces in gaining acceptance in the United States.
Eye Banks’ Key Role
Dr. Carlson, however, has worked out a solution to the eye bank issue.
In North Carolina. About 3 years ago, Ashiyana Nariani, MD, MPH, Dr. Carlson’s fellow, introduced him to PDEK, which she had learned from Dr. Agarwal. Drs. Carlson and Nariani worked closely with Miracles in Sight Eye Bank in Winston-Salem, North Carolina, to determine how the eye bank could prepare PDEK grafts effectively and predictably in order to minimize waste of corneal donor tissue and endothelial cell loss. The result, said Dr. Carlson, is that “Miracles in Sight Eye Bank has done a tremendous job preparing tissue that is preloaded and prestamped.”
In Florida. Dr. Mahootchi adopted DMEK, due in large part to the availability of preloaded and prestamped tissue grafts prepared by The Lions Eye Institute for Transplant & Research (LEITR) in Tampa. When he wanted PDEK tissue, he said, “I approached LEITR, which was able to deliver the preloaded PDEK graft I used for the first case that I performed in December 2016.”
___________________________
1 Agarwal A et al. Br J Ophthalmol. 2014;98(9):1181-1185.
2 Agarwal A et al. Cornea. 2015;34(8):859-865.
3 Kumar et al. J Cataract Refract Surg. 2015;41:1535-1536.
4 Agarwal A et al. Can J Ophthalmol. 2017;52(5):519-526.
5 Dua HS et al. Clin Ophthalmol. 2015;9:1155-1157.
Continuing Innovation
Dr. Agarwal continues to investigate alternative ways to treat complex cases. In some situations, he combines PDEK with other novel techniques.
For example, when he has a PDEK case in which the iris is badly defective and the patient needs cataract surgery, he performs a “triple procedure” that combines a glued intrascleral intraocular lens (IOL) procedure with pupilloplasty and PDEK.1
“We first perform the glued IOL procedure because it helps secure the IOL fixation,” Dr. Agarwal said. “This technique was introduced in 2007 for sutureless scleral fixation of the IOL via transscleral haptic tuck in patients with absent or deficient capsular support.”
Next, Dr. Agarwal performs a new technique called the single-pass 4-throw pupilloplasty (SFT), which repositions the iris structure and reconstructs the pupil while imparting stability to the anterior chamber and preventing air diversion into the vitreous cavity.
“As the name suggests, a 10-0 prolene suture on a long-arm needle is passed through the iris tissue followed by creation of a loop with 4 throws around it that slide inside the eye,” Dr. Agarwal noted. “This creates a helical configuration that prevents the suture from opening up. The 4 throws create an intertwining of sutures that has a self-locking mechanism and prevents loosening of the suture loop.”
He added that this approach facilitates the PDEK procedure, completing the surgical repair. SFT also helps solve angle-closure glaucoma, if present, by opening the angle.
___________________________
1 Narang P et al. Cornea. 2015;34(12):1627-1631.
|
Dr. Agarwal is chairman and managing director of Dr. Agarwal’s Eye Hospital and Eye Research Centre in Chennai, India. Relevant financial disclosures: Jaypee: P; Mastel: P.
Dr. Carlson is professor of ophthalmology at Duke University School of Medicine in Durham, N.C. Financial disclosures: Alcon: L; iVeena: O; MED1: C,O; Staar: L; TearScience: C,L,O.
Dr. Colby is Louis Block Professor and Chair of the Department of Ophthalmology and Visual Science at the University of Chicago Medicine. Relevant financial disclosures: None.
Dr. Mahootchi is medical director of The Eye Clinic of Florida in Zephyrhills. Relevant financial disclosures: None.
Dr. Tan is the Arthur Lim Professor of Ophthalmology at the Singapore National Eye Centre and at the Duke-National University of Singapore Medical School. Relevant financial disclosures: Network Medical Products: P.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Agarwal Bausch + Lomb: S; Jaypee: P; Mastel: P; Sanoculus: C; Slack: P; Staar: C; Thieme: P.
Dr. Carlson Alcon: L; iVeena: O; MED1: C,O; Staar: L; TearScience: C,L,O.
Dr. Colby WL Gore and Associates: C.
Dr. Mahootchi Bausch + Lomb: C; Ellex: C; Imprimis: C.
Dr. Tan Eye Lens: C; Network Medical Products: P; Santen: C,L.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|