By Mike Mott, Contributing Writer, interviewing Christopher D. Conrady, MD, PhD, Harry W. Flynn Jr., MD, and Cason B. Robbins, MD
Download PDF
How should ophthalmologists treat endophthalmitis when it occurs after an intravitreal injection (IVI)? Now that IVIs are a common medical procedure in the United States, with more than 3 million injections occurring annually,1 this question has, understandably, gained traction with retina specialists.
Adapting to the IVI Era
The landmark Endophthalmitis Vitrectomy Study (EVS), published in 1995, demonstrated the visual benefit of pars plana vitrectomy (PPV) for patients who present with visual acuity (VA) of light perception or worse within six weeks of cataract surgery or secondary IOL implantation. It also confirmed the practice pattern of empiric broad-spectrum intravitreal antibiotics.2
It’s unclear whether the EVS guidelines can be routinely generalized to today’s IVI-related endophthalmitis. “In essence, we’re extrapolating practice guidelines from 30 years ago, which was well before we began regularly performing IVIs,” said Cason B. Robbins, MD, who has collaborated with Sharon Fekrat, MD, to build and query a large endophthalmitis database at the Duke University School of Medicine in Durham, North Carolina.
There’s no question that the EVS was a critically important study. “The EVS has been the ultimate randomized controlled trial for postcataract endophthalmitis,” said Christopher D. Conrady, MD, PhD, at the University of Nebraska Medical Center in Omaha. Now, however, many clinicians are advocating for changes in management of cases of endophthalmitis from IVIs. “This is due in part to what appears to be differences in the clinical picture between the inciting procedure, the pathogen’s aggressiveness, and long-term outcomes,” Dr. Conrady said. As a result, many retina specialists have developed their own strategies regarding treatment.
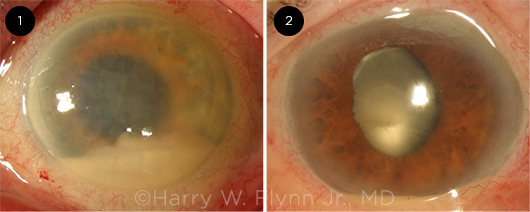 |
|
INFECTIVITY. Post-IVI endophthalmitis caused by (1) the virulent pathogen Streptococcus and (2) the comparatively indolent Staphylococcus epidermidis.
|
Considerations for Management
All retina specialists agree that treating post-IVI endophthalmitis starts with early diagnosis followed by prompt administration of broad-spectrum intravitreal antibiotics (including vancomycin and ceftazidime) via tap and inject or with vitrectomy, said Harry W. Flynn Jr., MD, at the Bascom Palmer Eye Institute in Miami.
That said, there are remaining management questions regarding cultures, the benefits of systemic antibiotics, the efficacy of early PPV, and the use of intravitreal corticosteroids. Factors to consider include the following.
Infectious or noninfectious? “Distinguishing between infectious or noninfectious endophthalmitis following an IVI can be clinically challenging,” said Dr. Flynn (see “Is It Infectious?”). “Less inflammation might signal a noninfectious case, but it might also be due to low pathogen virulence or a bacterial load that’s just small enough to evade detection.” And because delay in treatment could result in irreversible loss of vision, ophthalmologists typically have a low threshold for administering broad-spectrum intravitreal antibiotics, Dr. Flynn said.
For infectious endophthalmitis, long-term visual outcomes vary by causative organism. “For instance, streptococcal species are typically rapidly progressive and proinflammatory,” Dr. Conrady said. As a result, he said, they typically lead to worse outcomes compared to other organisms “such as more indolent staphylococcal infections that, while still consequential, don’t have the same destructive outcomes.”
And thanks to Dr. Flynn and his research team, there are emerging data to affirm these clinical observations, said Dr. Conrady. Thus, he said, “I think we should begin to reevaluate [the strategy] in which initial treatment is dictated by presenting visual acuity” and, instead, base protocols on overall clinical features and isolated pathogens.
From theory to practice. To put this into clinical perspective, for example, if a patient were to present for evaluation with endophthalmitis from Streptococcus three days after cataract surgery and with 20/80 vision, the EVS would advise a tap and inject, said Dr. Conrady. Today, however, clinicians are well aware of how virulent that pathogen is, he said. “That’s an eye at high risk of being totally lost.” Indeed, Dr. Flynn noted, “We know that an eye infected with the Streptococcus pathogen has a 90% chance of blindness.”
As a result, Dr. Conrady said, more aggressive treatment such as immediate vitrectomy and broad-spectrum antibiotics may be warranted to reduce the inflammatory and pathogen load within the vitreous.
Adjunctive role of systemic antibiotics. Although the use of intravitreal antibiotics is paramount in treating post-IVI endophthalmitis, there’s less consensus as to whether their systemic counterparts are helpful, said Dr. Flynn. “And because the typical patient you’re treating is elderly, possibly diabetic, or presenting with multiple other medical problems, you do want to be especially careful that you aren’t creating a new set of problems, be it adverse events or the need for hospitalization.” Given these potential risks, he said, he personally doesn’t use systemic antibiotics in this population of patients with post-IVI endophthalmitis.
This is an instance in which prospective data would be helpful, said Dr. Conrady. “We have emerging pharmacokinetics data related to oral fluoroquinolones and vitreous penetration,” he said.3 And today’s fluoroquinolones, specifically moxifloxacin, have better penetration than the systemic medications used in the EVS, he said. “This has led some clinicians to advocate for a five- to seven-day course of oral moxifloxacin, in addition to intravitreal antibiotics.”
Do ophthalmologists have any promising data that would support or warrant such a widespread change in treatment patterns? “No, it hasn’t been widely studied,” said Dr. Conrady. But it’s possible that an oral antibiotic might extend the antimicrobial effect of its intravitreal counterpart and reduce the need for additional injections in some cases, he noted. “It’s just that we need the results from a randomized control trial for ophthalmologists to hang their hat on.”
Moreover, as Dr. Flynn pointed out, the use of fluoroquinolones cannot be undertaken lightly: “Fluoroquinolones can cause significant side effects, including tendon injury/rupture and aortic dissection.”
Early vitrectomy. The EVS reported that early PPV (performed within six hours of presentation) was most beneficial in eyes with light perception vision.2 But does early PPV benefit certain eyes with better than light perception vision?
“Since the EVS was published, vitrectomy surgery has become safer and more refined,” said Dr. Robbins. “For example, we now have widefield visualization and smaller-gauge instrumentation. So in 2021, it might be that early PPV for the initial treatment of post-IVI endophthalmitis may offer visual benefit and better outcomes even for eyes with visual acuity that is hand motion or better.”
Although it’s difficult to draw definitive conclusions from a retrospective analysis, said Dr. Robbins, nine-year surgical data from his work with Dr. Fekrat and the team at Duke did show their surgeons’ willingness to perform PPV earlier than indicated by the EVS—with a VA threshold of around counting fingers.4 The Duke researchers also studied a specific cohort of individuals with endophthalmitis after IVI and reported that those with VA of hand motion or worse were more likely to undergo subsequent PPV after initial presentation, suggesting that these patients had more recalcitrant disease and may benefit from earlier vitrectomy.5
Don’t delay! Many U.S. retina specialists have changed their treatment threshold, said Dr. Conrady. But whether the strategy is tap and inject and/or PPV, the most important message to surgeons is to “get the antibiotics in the eye without delay,” as the smallest interruption in treatment can have substantial consequences.
“This continues to be especially important during the pandemic,” he said. “Because timing is essential, you have to be aware of OR availability. If you foresee any delays, for example, consider tap and injecting in the interim to avoid delaying administration of intravitreal antibiotics while you await available OR time.”
Using corticosteroids. Whether or not to use oral, topical, and/or intravitreal corticosteroids in conjunction with antibiotics is also up for debate, said Dr. Flynn. “At Bascom Palmer, we use intravitreal corticosteroids for managing patients with post-IVI endophthalmitis because we feel that it provides us with the best ability to reduce inflammation in severe cases.”
In general, Dr. Flynn recommends that clinicians use their best judgment and avoid indiscriminate use of systemic steroids. For example, his department avoids systemic corticosteroids because so many presenting patients are diabetic, and the potential side effects of these medications include blood sugar elevation.
Intravitreal corticosteroid use “remains a controversial topic,” said Dr. Conrady. On one hand, he noted, “you don’t typically treat infections with corticosteroids for fear of making the infection worse.” But on the other, “We’re dealing with cells that don’t re-generate, so on top of battling the infection, we most likely need to reduce the associated inflammation, because it, too, is likely driving pathological changes within nonregenerative retinal tissue.”
A few randomized control trials have investigated the role of intravitreal corticosteroids in the management of endophthalmitis, but the results are mixed.6
“In one study, they help; in another, they fail to add any benefit,” Dr. Conrady said. “We need more data, especially data that consider corticosteroid half-life and route of administration.” Intravitreal dexamethasone, for example, has a half-life of hours rather than of days to weeks, which is typically how long inflammation secondary to endophthalmitis lasts, said Dr. Conrady. “So maybe we’re not treating the inflammation aggressively enough, since the corticosteroid effect might have already cleared itself from the vitreous before it could have any meaningful impact,” he added.
Is It Infectious?
Both endophthalmitis and noninfectious inflammation may occur following anti-VEGF injections. Although the primary differentiating factors are the severity of the inflammation and the timing of the symptoms, there may be some overlap between these two categories.
|
Characteristic
|
Infectious Endophthalmitis
|
Noninfectious Inflammation
|
|
Presentation
|
Usually 24-48 hours
|
Usually several days but highly variable; may be within 24 hours
|
|
Pain
|
Usually mild/moderate; can be severe
|
Usually none; may be mild
|
|
Visual Acuity
|
May be severely decreased
|
Mildly to moderately decreased
|
|
Corneal Edema
|
May be moderate or severe
|
Generally none or mild
|
|
Anterior Chamber
|
Moderate to severe cell and flare
|
Mild cell and flare
|
|
Fibrin
|
Generally present
|
Generally absent
|
|
Hypopyon
|
Often present
|
Generally absent
|
|
Vitreous
|
Moderate to severe cells
|
Mild to moderate cells
|
|
Management
|
Vitreous sampling, intravitreal antibiotics
|
Topical steroids; possible cycloplegics
|
|
Prognosis
|
Depends on organism; generally poor
|
Generally good
|
|
Source: Adapted from Schwartz SG et al. J VitreoRetin Dis. 2019;3(1):42-44.
|
What’s Next?
One missing piece for fine-tuning the treatment of endophthalmitis specific to IVIs: a prospective analysis similar to the EVS. But that may not become a clinical reality in the near future, said Dr. Robbins. Dr. Flynn agreed: “Whereas postcataract endophthalmitis is relatively common, post-IVI endophthalmitis is relatively rare. It would be difficult to recruit and randomize patients to alternative treatment strategies.”
Modalities now on the horizon may eventually guide management of post-IVI endophthalmitis. “Rapid, point-of-care vitreous proteomic and genomic analyses, for example, are quite exciting,” said Dr. Robbins. “With more research, we might be able to provide a personalized approach with the identification of specific inflammatory mediators that comprise an inflammatory signature of a patient in order to tailor management with targeted therapies.”
Regardless of the approach, any advances will require partnerships among retina specialists across the country and around the world, Dr. Conrady said. “Despite the hurdles ahead, it’s important that we move forward in collaborative efforts because endophthalmitis remains one of our most devastating complications of intraocular procedures and surgeries.”
___________________________
Further Reading. Reyes-Capo DP et al., for the Endophthalmitis/Anti-VEGF Study Group. Trends in endophthalmitis associated with intravitreal injection of anti-VEGF agents at a tertiary referral center. Ophthalmic Surg Lasers Imaging Retina. 2021;52(6):319-326.
___________________________
1 Dhoot DS et al. Ophthalmic Surg Lasers Imaging Retina. 2021;52(6):312-318.
2 Endophthalmitis Vitrectomy Study Group. Arch Ophthalmol. 1995;113(12):1479-1496.
3 Conrady CD, Yeh S. Pharmaceutics. 2021;13(8);1224.
4 Feng HL et al. Ophthalmol Retina. 2020;4(6):555-559.
5 Soundararajan S et al. J VitreoRetin Dis. Published online July 28, 2021.
6 Brundrett A et al. Curr Ophthalmol Rep. 2018;6:105-114.
___________________________
Dr. Conrady is a retina and uveitis specialist at the University of Nebraska Medical Center in Omaha. Relevant financial disclosures: None.
Dr. Flynn is the J. Donald M. Gass Distinguished Chair and professor of ophthalmology at the Bascom Palmer Eye Institute in Miami. Relevant financial disclosures: None.
Dr. Robbins is an ophthalmology resident at the Duke University School of Medicine in Durham, N.C. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Conrady Heed Ophthalmic Foundation: S.
Dr. Flynn None.
Dr. Robbins None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|