By Alex Im, BS, Albert Y. Cheung, MD, and Elizabeth Yeu, MD
Edited By: Bennie H. Jeng, MD
Download PDF
Fuchs dystrophy is a slow, progressive degeneration of the corneal endothelium, leading to stromal edema. The edema can cause symptoms such as blurry vision, eye pain, and light sensitivity. Signs and symptoms can begin to appear in the fourth decade of life, although the typical onset is between the fifth and seventh decades. In developed nations, Fuchs dystrophy is one of the most common indications for corneal transplantation.1
Genetics
Fuchs dystrophy is inherited in an autosomal dominant pattern, with a strong predilection for women (3:1 ratio); evidence also suggests a higher prevalence in white populations. The disorder’s genetic basis and pathophysiology are multifactorial, with recent studies illuminating various contributing genes.
One of these genes, COL8A2, is responsible for the α2 subtype of collagen VIIIA, and a point mutation in this gene is responsible for altering a key component of Descemet membrane in the early-onset subtype of Fuchs.1,2 The SLC4A11 gene, associated with a late-onset Fuchs subtype, encodes for the densely populated endothelial membrane borate pump (transportation of water and ammonia), responsible for maintaining deturgescence. The TCF4 gene encodes for the E2-2 protein, which belongs to a family of transcription factors responsible for cell growth and differentiation. Other associated genes include ZEB1, AGBL1, KANK4, LAMC1, ATP1B1, LOXHD1, and DMPK.1,2
 |
|
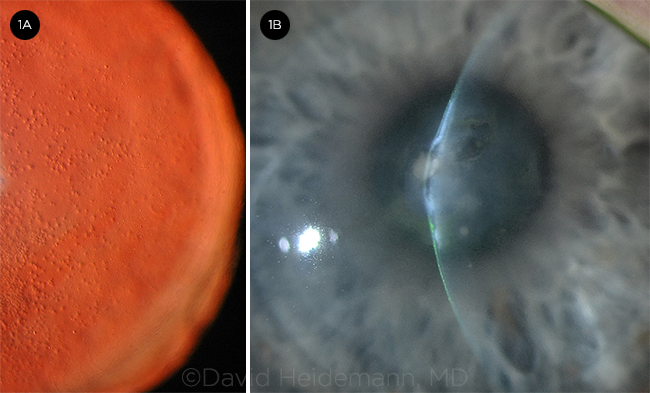
SLIT-LAMP PHOTOS. Eyes with Fuchs demonstrating (1A) cornea guttae highlighted with retroillumination and (1B) severe central bullous keratopathy (microcystic edema and bullae) and stromal fibrosis.
|
Diagnosis
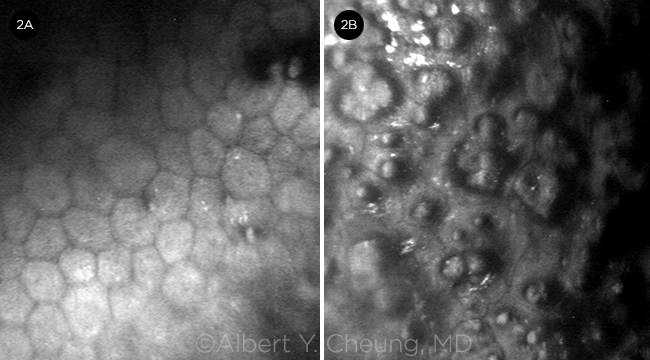
The diagnosis of Fuchs is made primarily based on a comprehensive exam, as there is no definitive diagnostic test. A careful slit-lamp exam, including retroillumination, can identify central guttae (excrescences in Descemet membrane; Fig. 1A). The guttae may spread peripherally and may coalesce, forming a beaten metal appearance. Edema may appear as a fine gray haze (best seen with sclerotic scatter), and it may progress to fine vertical wrinkles (striae), overt Descemet folds, and microcystic epithelial edema and bullae (Fig. 1B). A pachymeter may be useful in identifying corneal edema, particularly if baseline pachymetry is available for the patient. Specular microscopy can also be used to determine the number and quality of endothelial cells (Fig. 2).
Staging. Endothelial degeneration progresses slowly over the course of 10 to 30 years and can be clinically categorized by three stages of symptoms and signs.
- The initial stage is characterized by the presence of central guttae. Often asymptomatic at this stage, guttae can lead to symptomatic glare from higher-order aberrations and backscatter.
- The second stage is marked by the accumulation of fluid in the stroma and epithelium, producing blurry vision, halos around lights, and eye pain. Symptoms may be worse in the morning as a result of decreased evaporation of corneal fluid when the eyes are closed during sleep.
- The final stage is marked by loss in visual acuity (VA) and is remarkable for the development of avascular subepithelial fibrous scarring between the epithelium and Bowman membrane (best viewed with tangential illumination), peripheral superficial corneal neovascularization, and a reduction in edema.
Treatment
Medical therapy. In the early course of the disease, medical management aims to remove excess fluid from the cornea by using topical hypertonic saline ointment/solutions (e.g., 5% sodium chloride), dehydrating the cornea with a blow dryer in the morning or throughout the day (at low heat and kept at an arm’s distance to avoid burning/damage), and reducing intraocular pressure. Bandage contact lenses may decrease symptoms from painful bullae or prevent recurrent erosions.
Keratoplasty. Surgery (see table below) is indicated when symptoms warrant intervention, often when there is a decrease in VA or discomfort from epithelial edema or erosions.
PK vs EK. Historically, penetrating keratoplasty (PK, full-thickness keratoplasty) was a common treatment. PK is now reserved for corneas with combined pathologies such as Fuchs in the setting of symptomatic anterior corneal scarring, stromal dystrophy, or keratoconus.
The currently preferred approach for symptomatic Fuchs is partial-thickness endothelial keratoplasty (EK), which selectively replaces the dysfunctional corneal endothelium. These procedures include Descemet stripping automated endothelial keratoplasty (DSAEK) and Descemet membrane endothelial keratoplasty (DMEK; Fig. 3).
Compared with traditional PK, EK has the advantages of superior visual outcomes, faster recovery, fewer intraoperative and suture-related complications, lower graft rejection and failure rates, and greater tectonic integrity. Reviewing long-term outcomes in Fuchs eyes, Woo et al. found that PK had worse graft survival rates (73.5%) than DMEK (98.7%) or conventional DSAEK (96.2%).3 Additionally, PK had a higher graft rejection rate (14.1%) than DMEK (1.7%) or conventional DSAEK (5.0%).
DMEK. Series reported in the literature4-6 show that DMEK provides an average best-corrected VA (BCVA) of 20/25 in 50% to 80% of Fuchs and pseudophakic bullous keratopathy cases at six months. DMEK achieves 20/20 or better in at least 40%, although Fuchs eyes tend to attain better results.
The DETECT trial showed that DMEK yields superior visual results compared with ultrathin DSAEK and has similar endothelial and complication outcomes.7 Despite the superior clinical outcomes of DMEK (better BCVA, faster visual recovery, decreased rejection rates), DSAEK is still performed more commonly in the United States. This is because DMEK has a steeper learning curve and higher postoperative graft dehiscence rate.
Advanced DSAEK. Thinner DSAEK grafts in the ultrathin (50-100 μm) and, more recently, nanothin range (≤50 μm) have shown promising results that are close, if not comparable, to DMEK in terms of BCVA (53% achieving 20/20 at five years for ultrathin, and 57% achieving 20/20 at one year for nanothin in certain series).8,9 Although DMEK still allows for quicker visual recovery than the new DSAEK approaches, ultrathin and nanothin DSAEK provide EK surgeons with a viable alternative that affords the same comfort and predictability as conventional DSAEK. Intraoperatively, the tissue behaves similarly in eyes with complex anterior segment anatomy as it does for routine Fuchs cases.
DWEK/DSO. Certain Fuchs patients may benefit from descemetorhexis without endothelial keratoplasty (DWEK), also known as Descemet stripping only (DSO). These are patients with symptomatic central guttae or edema and clear peripheral cornea with an endothelial cell count (ECC) of at least 1,000 cells/mm2. In this technique, the central 4 mm of Descemet and endothelium is removed by means of descemetorhexis. Resolution of central corneal edema occurs through migration and regeneration of peripheral endothelial cells after a mean of three months.
While reported clearance rates range from 63% to 100%, the use of ripasudil, a topical rho-associated protein kinase (ROCK) inhibitor, may improve endothelial proliferation and corneal clearance.10 DSO eliminates graft complications, rejection, and the need for long-term postoperative corticosteroids.
However, because experience is limited with this procedure compared to the others, careful patient selection is important. DSO may be rescued with a DMEK surgery if the cornea fails to clear.
 |
|
SPECULAR MICROSCOPY FINDINGS. (2A) Polymegethism and occasional guttae compared with (2B) severe polymegethism and significant guttae.
|
Other Procedures
For Fuchs patients with corneal edema and recurrent erosions who are not candidates for keratoplasty, anterior stromal micropuncture may be applied in focal areas of painful bullae. This technique can also be used after EK for residual bullae (i.e., in the areas of bare stroma if there is a mismatch between the descemetorhexis and the EK graft).
Another option for treating bullous keratopathy may be corneal cross-linking, which can improve symptoms, vision, and/or pachymetry in certain cases.11 Although improvement can be seen in the first month, regression may occur over the ensuing months.
 |
|
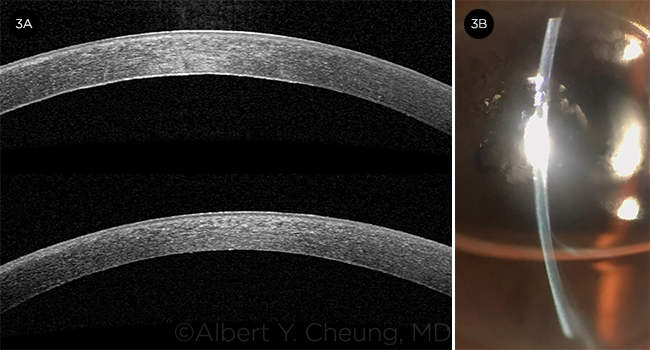
BEFORE AND AFTER. Anterior segment OCT of Fuchs eye (3A, top) before and (3A, bottom) one week after DMEK surgery. Note the cornea edema with visible folds, thickened Descemet, and guttae in the top image. The cornea is appreciably thinner in the bottom image; it is also impossible to detect the graft, as it is an anatomic replacement. (3B) Slit-lamp photograph one day after DMEK surgery with a 60% gas fill shows that the cornea has already thinned.
|
EK With Cataract Surgery
In Fuchs patients with a visually significant cataract, cataract surgery can be performed alone, at the same time as EK, or in a staged process based on which pathology (cornea or cataract) is more symptomatic or which order the ophthalmologist is most comfortable with. When the cataract appears to account for the visual decrease, cataract surgery may be considered earlier when there is more endothelial reserve. An ECC less than 1,000 cells/mm2 or corneal thickness greater than 640 μm should raise concern about the possibility of corneal decompensation with intraocular surgery.12
Cataract technique and lenses. During cataract surgery in Fuchs, an effort should be made to replenish dispersive ophthalmic viscosurgical devices (OVDs) during nuclear disassembly to give continued protection to the endothelium. Femtosecond laser may decrease the phacoemulsification energy needed and the subsequent endothelial damage.
Hydrophilic IOLs should be avoided, as they can develop hydroxyapatite deposition from the gas or air fill if an EK is needed in the future. Multifocal IOLs should also be avoided in Fuchs because of their diffractive optics and reduced image quality. In patients who are expected to undergo EK in the future, the surgeon may select the IOL power for postsurgical myopia to compensate for EK-induced hyperopia.
Combined surgery. For combined cataract surgery and EK, the surgeon should adjust the paracentesis sites for the EK (postoperatively, a superior paracentesis allows for selective gas removal, while an inferior paracentesis allows for aqueous removal). A smaller anterior capsulorrhexis (~4.5 mm) will minimize anterior displacement of the IOL during the anterior chamber shallowing and filling that occurs during EK. A cohesive OVD is utilized to ensure complete removal, and phacoemulsification can be performed above the iris plane to protect the posterior capsule without concern for the endothelium. IOL power selection should account for the expected hyperopic shift of approximately 0.50 to 0.75 D for DMEK and 1.00 to 1.25 D for DSAEK. Eyes with greater corneal edema (thicker central pachymetry) as well as flatter and more oblate posterior corneal surfaces may have greater-than-expected hyperopic shifts.
Surgical Treatments
|
Procedures
|
Abbreviation
|
What Is It?
|
Thickness of Graft Tissue
|
|
Penetrating keratoplasty
|
PK
|
Full-thickness replacement of the patient’s cornea with a donor corneal graft
|
Full thickness
|
|
Descemet stripping automated endothelial keratoplasty
|
DSAEK
|
Selective removal of the patient’s Descemet membrane and endothelium followed by transplantation of a donor graft composed of corneal stroma (variable thickness), Descemet membrane, and endothelium
|
100-200 μm
|
|
Ultrathin Descemet stripping automated endothelial keratoplasty
|
UT-DSAEK
|
50-100 μm
|
|
Nanothin Descemet stripping automated endothelial keratoplasty
|
NT-DSAEK
|
≤50 μm
|
|
Descemet membrane endothelial keratoplasty
|
DMEK
|
Selective removal of the patient’s Descemet membrane and endothelium followed by transplantation of a donor graft composed of Descemet membrane and endothelium
|
0-15 μm
|
|
Descemetorrhexis without endothelial keratoplasty/Descemet stripping only
|
DWEK/DSO
|
Selective removal of the patient’s Descemet membrane and endothelium; no subsequent donor graft transplantation
|
No graft tissue
|
Future Options
Kinoshita and colleagues have published results of a clinical trial using cultured human corneal endothelial cells (CECs) supplemented with a ROCK inhibitor in eyes with endothelial disease, including Fuchs.13 After mechanical removal of abnormal extracellular matrix on the Descemet membrane, the cultured CECs were injected into the anterior chamber, and the patients were positioned face down for three hours. Results were promising, with an increase in CEC density, decrease in pachymetry, and clearing of the cornea by 24 weeks; at two years, corneal transparency was maintained, and no serious adverse events were noted.
Conclusion
Fuchs dystrophy is an endothelial degeneration that results in progressive stromal edema. Endothelial keratoplasty (DSAEK, DMEK) currently remains the preferred treatment for symptomatic Fuchs; however, newer surgical techniques such as DSO may benefit specific patients. Future advances with cultured human CECs eventually may change the treatment paradigm.
___________________________
For more information, see “Genetic Disorders of the Cornea: Preventing Surgical Surprises.”
___________________________
1 Nanda GG, Alone DP. Mol Vis. 2019;25:295-310.
2 Sarnicola C et al. Eye Contact Lens 2019;45(1):1-10.
3 Woo JH et al. Am J Ophthalmol. 2019;207:288-303.
4 Trindade BLC, Eliazar GC. Clin Ophthalmol. 2019;13:1549-1557.
5 Guerra FP et al. Ophthalmology. 2011;118(12):2368-2373.
6 Rodriguez-Calvo-de-Mora M et al. Ophthalmology. 2015;122(3):464-470.
7 Chamberlain W et al. Ophthalmology. 2019;126(1):19-26.
8 Madi S et al. Cornea. 2019;38(9):1192-1197.
9 Kurji KH et al. Cornea. 2018;37(10):1226-1231.
10 Garcerant D et al. Curr Opin Ophthalmol. 2019;30(4):275-285.
11 Ucakhan OO, Saglik A. Case Rep Med. 2014;463905.
12 American Academy of Ophthalmology. Cataract/Anterior Segment Panel. Cataract in the Adult Eye Preferred Practice Pattern. aao.org/preferred-practice-pattern/cataract-in-adult-eye-ppp-2016.
13 Kinoshita S et al. N Engl J Med. 2018;378:995-1003.
___________________________
Mr. Im is a second-year medical student at Eastern Virginia Medical School (EVMS) in Norfolk, Va. Drs. Cheung and Yeu are cornea specialists at Virginia Eye Consultants/CVP in Norfolk and are on faculty at EVMS’ Department of Ophthalmology. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Cheung Alcon: C; Eye Bank Association of America: S.
Mr. Im None.
Dr. Yeu Alcon; C; Allergan: C; Avedro: C; Bausch + Lomb/Valeant: C; Beaver Visitec: C; Bruder: C; EyePoint: C; Glaukos: C; iOptics: C; Guidepoint: C; J & J Vision: C; Lensar: C; Kala: C; Novartis: C; Ocular Science: C; Ocular Therapeutix: C; Ocusoft: C; Omeros: C; Oyster Point Pharma: C; ScienceBased Health: C; Shire: C; Sight Sciences: C; SightLife: C; Sun: C; TissueTech: C; Topcon: C; TearLab: C; TearScience C; Zeiss: C.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|