This article is from May 2012 and may contain outdated material.
European cataract surgeons face the same overarching challenge that their U.S. colleagues do with presbyopic IOL patients: managing high expectations. Unlike U.S. ophthalmologists, however, they have a wider range of lens options to offer their patients.
Here’s a closer look at the features and optics of some popular lenses.
American cataract surgeons often find themselves watching the European market for the “next big thing” in intraocular lenses (IOLs). Even though the designs may originate in the United States, marketing approval and widespread use typically get a head start abroad.
But no matter where a cataract surgeon practices—London, England, or New London, Conn.—the challenges of achieving high-quality vision and patient satisfaction, particularly with presbyopic patients, are the same. What’s the best solution, if any, for intermediate vision? Which IOL has the fewest problems with halos and glare at night?
Here’s a look at three of the newer multifocal IOLs in Europe, along with an update on some other intriguing designs in the pipeline.
IOL Design Variables
The quality of the retinal images produced by a multifocal IOL depends on multiple variables, said Kenneth J. Hoffer, MD, the Santa Monica, Calif., surgeon whose credentials in ophthalmic optics include development of the Hoffer Q formula for calculating IOL power. These variables include internal aberrations caused by the shape of the lens as well as the interaction between pupil size and the IOL’s refractive or diffractive characteristics, said Dr. Hoffer, a clinical professor of ophthalmology at the University of California, Los Angeles.
Diffractive and refractive designs. The most obvious of the variables is the optical approach used to achieve multifocality. Although many refinements have occurred since multifocal IOLs became commercially available, the two basic designs are diffractive and refractive.
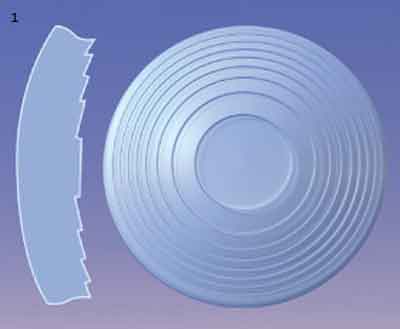
Diffractive lenses employ closely spaced concentric rings on one of the lens surfaces to split incoming light into multiple beams; they add together in phase at a predetermined point on the optical axis for near focus, while the overall curvature of the lens provides the distance focus (Fig. 1). The spacing and number of the rings, as well as their step heights, vary by manufacturer and model.
Refractive IOLs use zones of different optical powers. Designs include bifocal lenses, which present two simultaneous images; or, more commonly, multiple annular zones of alternating distance and near focus that depend on pupil size to create the appropriate aperture for the desired visual task (Fig. 2).
Different designs, different challenges. Different designs, different challenges. One critical factor is the way in which incoming light is distributed to each of the focal points of an IOL.
“Back when 3M came out with their diffractive multifocal IOL in the 1990s, we learned something important. We learned that 20 percent of the light going into an eye with this type of IOL disappeared. It was just gone,” Dr. Hoffer said. “So you’re only left with 40 percent of the incoming light for distance and 40 percent for near. The challenge is to try to come up with lenses that don’t waste any of that light.”
On the other hand, certain optical issues are specific to refractive lenses, according to Damien Gatinel, MD, assistant professor and head of anterior segment and refractive surgery at the Roths-child Ophthalmology Foundation in Paris. “The way refractive IOLs provide multifocality is based on the juxtaposition of refractive zones of different power throughout the surface of the IOL. Therefore, by design, refractive IOLs are pupil dependent and generate some higher-order aberrations through which the depth of focus is increased,” he said. One difficulty presented by these designs is that the light conditions—and, thus, the pupil size—might not match the visual task. For example, in many designs, the central zone is assigned to distance; thus, if the pupil constricts in bright light, the patient will lose near focus.
|

|
|
DIFFRACTIVE IOL. (1) Closely spaced stepped rings diffract incoming light into near and distance focal points. REFRACTIVE IOL. (2) Refractive designs use zones of different optical powers, most commonly in alternating rings of near and far foci, to achieve multifocality.
|
Three Approaches to Optics Problems
Three popular presbyopia-correcting IOLs currently used in Europe are the AT LISA (previously known as the Acri.LISA; Carl Zeiss Meditec), the Lentis Mplus (Oculentis), and the FineVision (PhysIOL).
“All three are perfect for microincisional cataract surgery, as they are implantable through 1.8-mm incisions,” said Sheraz M. Daya, MD, consultant and medical director at the Centre for Sight in East Grinstead, United Kingdom. And each design represents a different solution to the central dilemma of multifocal lens design: how to divide up incoming light to optimize distance, intermediate, and near vision.
■ AT LISA: A 65/35 Split
The single-piece, aspheric AT LISA, available in toric and nontoric versions, is a refractive-diffractive hybrid lens with 3.75 D of near add (Fig. 3). The lens directs light to one focal point for distance vision and another for near vision, requiring the patient to neuroadapt to the presence of two simultaneous images.
AT LISA favors distance. The lens has stepped, concentric rings of alternating power that, independent of pupil size, divide usable light 65 percent for distance and 35 percent for near.1 The concentric diffractive rings on the posterior IOL surface are divided into main zones and phase zones; the latter zones assume the function of the steps of diffractive IOLs. AT LISA rounds off the sharp edges of the optical steps on its surface in order to reduce the internal reflections that cause glare and halos at night. To improve retinal image quality, the posterior surface is shaped to neutralize 0.11 µm of the optical system’s positive spherical aberration.
“My favorite multifocal IOLs are diffractive, and they represent a growing percentage of my total IOL share,” said Dr. Gatinel. “In my opinion, diffractive IOLs provide more consistent results on average [than refractive lenses]. I particularly like IOLs that favor distance vision in mesopic conditions. This is obtained with the AT LISA through constant energy split.”
High patient satisfaction, but some surprises. Patient satisfaction with the AT LISA is “very high, with remarkable near and distance vision—20/15 in many cases,” said Dr. Daya, who has implanted roughly 500 of the AT LISA IOLs, including the toric version.
Intermediate vision, however, can be problematic for some patients, he said. Objects at intermediate distances appear blurry because the retinal image is a combination of the defocused distance and near images. In clinical studies, patients with the nontoric version of AT LISA had binocular central intermediate acuity, at 70 cm, of 0.27 to 0.34 logMAR (approximately 20/38 to 20/45).2-4 Visser et al. noted that about a third of subjects implanted with the toric AT LISA reported moderate to severe visual difficulty at computer working distance.5
Toric vs. nontoric popularity. The toric AT LISA continues to be popular in the European market, but use of the nontoric version—previously a mainstay for European surgeons—has been slowing as other multifocal options have entered the market.
“I used the [nontoric] AT LISA extensively a couple of years ago,” said Erik L. Mertens, MD, medical director and eye surgeon at Medipolis in Antwerp, Belgium. “However, I was not always happy with the refractive stability and outcome afterward. Because it is a thin lens, its position within the bag is not always the same. That sometimes led to refractive surprises that required a touch-up afterward.” Some of his patients also complained of halos and glare and said that night driving was a challenge.
Dr. Mertens said he still considers the toric AT LISA an option. “It is a thicker lens and is more stable in the bag.” In a 45-eye study of the toric AT LISA, the mean IOL misalignment three months postoperatively was 2.3 ± 2.0 degrees (median 2.0 degrees; range 0 to 7 degrees).5
 |
|
AT LISA. (3) This multifocal lens is available in both toric (shown here) and nontoric models.
|
■ Lentis Mplus: Bifocal Design
Instead of having rotational symmetry like other multifocal IOLs, the Lentis Mplus takes its design cues from Benjamin Franklin’s bifocals. It is a one-piece zonal refractive lens with plate haptics and two refractive segments: a large aspheric distance-vision zone and a sector-shaped zone with 3 D of near add, embedded on the posterior surface. The add sector is the only area of the lens that directs light to a near focal point; the remainder of the optic acts as a monofocal IOL for distance vision.6 It is the first commercially available IOL based on this nonrotational bifocal concept.1
Good predictability. In addition to dedicating most of the lens to distance vision, the design is intended to minimize two common problems of multifocal IOLs: a loss of distance contrast sensitivity at night, and photic phenomena like glare and halos.
Dr. Mertens said that once he had shifted away from using the regular AT LISA, he added the Mplus to his practice. “It gives me very good predictability in refractive outcome, without major side effects and with very good distance vision.” He also prefers to use the Mplus toric IOL for his presbyopic patients with astigmatism.
Better for night driving. Dr. Mertens said that only a fraction of his Mplus patients complain of problems with driving at night. “The typical glare and halos are not present,” he said. In one published trial, 6.2 percent of patients reported moderate halos, 12.5 percent moderate glare, and 15.6 percent moderate night-vision problems. None of the patients rated these photic phenomena as severe.7,8
He added that some patients experience short-term bouts of double vision, in which they might see a clear road sign and a secondary, shadowy one. “With the bifocal design, you are seeing distance and near focus simultaneously. If the brain cannot suppress one of the two foci, a patient will experience double vision.” The good news is that this tends to fade in a couple of months after surgery, as neuroadaptation takes place, he said.
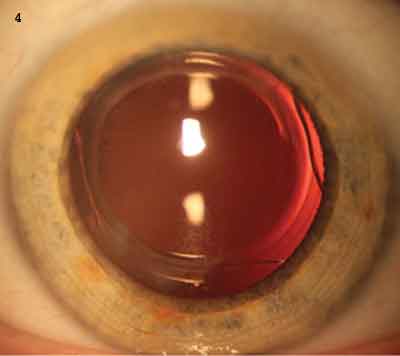
Optical aberrations may occur. However, according to Dr. Daya, patients sometimes report that bright lights, such as car headlights at night, cause a downward starburst or flare that washes out their view of the road ahead. He said that this phenomenon occurs because the lens induces the optical aberration of coma.1
For this reason, Dr. Daya said, “I choose to turn the lens upside down so that any flare experienced goes upward. This enables patients to drive without any flare from headlights interfering with their ability to see the road at night.” (See Fig. 4.)
Placement of the add segment doesn’t matter. Although it might seem intuitive that positioning the IOL in this way could cause problems with reading, Dr. Hoffer said he doubts that it matters. He has considerable experience with bifocal IOL design, having invented a split bifocal lens back in 1982 that was implanted in several patients, although never developed commercially.9 He said that the position of the near add matters with eyeglasses but not when the lens is inside your eye.
“The reason the bifocal is at the lower part of your eyeglasses is that your eye naturally looks down when you’re looking at a book. The add is there because the eye moves in that direction to read,” Dr. Hoffer explained. “But when you place a split bifocal lens inside the eye, it doesn’t make any difference what direction the eye is looking. The reading part will always pre-sent a near image to your retina no matter where you look. Thus, the add segment can sit superiorly, temporally, or nasally. It doesn’t make any difference. There is no ‘upside down.’”
Good corneal optics are essential. Dr. Daya said that the Mplus has the advantage of being “fairly forgiving” of slight positional errors. However, he continued, “It is vital that patients have good corneal optics. Any preexisting asymmetric corneal aberrations such as coma may not combine well with the optics of the Mplus, with [the result of] poor vision at all ranges. For this reason, all patients need corneal topography measurement preoperatively, preferably with a device that can provide information on cornea- derived aberrations.”
Lower near vision for some patients. Dr. Daya has implanted approximately 250 Mplus IOLs, and he reports that most patients are very satisfied. But about 10 percent require glasses for reading, he said, leading him to conclude that “near vision with the Mplus is not always good. In terms of patient selection, this lens is, in my experience, better for hyperopes.”
His experience echoes the findings in a report by Muñoz and colleagues late last year. Six months after surgery, only one patient out of 32 required spectacles for intermediate tasks (at 70 cm), but four of the 32 needed spectacles often or always to read.6
 |
|
LENTIS MPLUS. (4) This IOL has been implanted upside down to keep flares from headlights from interfering with the patient’s view of the road in night driving.
|
■ FineVision: Three Focal Points
The FineVision trifocal diffractive IOL was designed to provide patients with intermediate acuity by using the optical property of constructive interference to capture light energy that other diffractive IOLs lose. In addition to the distance correction, the FineVision lens has 3.5 D power for near and 1.75 D for intermediate vision. It was designed to “overcome the ‘gap’ that is encountered with the bifocal diffractive designs for that specific [intermediate] distance,” said Dr. Gatinel, who was part of the team that created the lens’ optical design.
FineVision’s anterior surface combines two diffractive profiles (one for distance and near, and the other for distance and intermediate), which are microlathed in an alternating pattern from center to periphery. In the same space where other multifocal IOLs have 12 or fewer optical steps, this IOL has more than 30 (Fig. 5).
Constructive interference captures more light. The designers developed computer models of how diffracted light behaves at the IOL surface. The computer then created iterative math equations that determined the shape and spacing of the diffractive peaks and valleys (kinoforms) on the FineVision’s anterior surface. The kinoforms are designed to cause constructive interference with the light diffracted by its two neighboring diffractive zones. The constructive interference increases the amount of light sent to each focal point, bench tests showed.10
The diffractive steps decrease in height from the center to the periphery, in order to shift more light to the far focal point as the pupil widens at night. For example, starting from the center, the distance from the bottom of the ninth step to the top of the 10th measures about 0.8 µm; but in the outermost zone, the last 10 diffractive steps are about 0.3 µm high.10
Results from bench testings. Data on the clinical performance of this IOL remain scarce. However, Dr. Gatinel’s group published a paper last year that describes the design and successful bench testing of the lens.10 In the report, they confirm the theoretical predictions about how Fine-Vision’s unusual surface profile would behave optically. The tests showed that:
- With a 3-mm aperture, the IOL distributed 42 percent of the incoming light energy to the distance focal point, 15 percent to intermediate, and 29 percent to near.
- The surface profile succeeded in reducing the amount of diffracted light lost to 15 percent. With previous diffractive IOLs, this figure is 20 percent.
- The lens became distance dominant at a bench-test “pupil” size of 4.5 mm.
Few optical side effects. As the FineVision is still relatively new, not all European surgeons have adopted it, but those who have used it predict that it could become popular quickly.
“I have to admit that I was skeptical in the beginning, when it came out,” Dr. Mertens said. “I thought, ‘Oh, it’s just another diffractive model.’ But I was extremely surprised to see so few side effects. Driving at night is no longer a problem for patients who have received this IOL. And when we measure higher-order aberrations, it’s amazing to see that we find virtually none that have been surgically induced.” As a result, he is using the Fine-Vision IOL for many of his presbyopic patients who do not have astigmatism.
Dr. Daya, who has implanted approximately 250 FineVision IOLs, also reports excellent results. “So far, all patients are completely independent of glasses. About 1 percent have required further astigmatic keratotomy or PRK for the correction of residual cylinder.”
 |
|
FINEVISION. (5) Diffractive steps have been designed to maximize light sent to each focal point by means of constructive interference of light waves.
|
Preparation for Patient Satisfaction
No matter how technically advanced an IOL may be, the key to patient satisfaction is careful preparation. “Multifocal IOL surgery is a procedure that starts before the actual surgery begins,” said Dr. Gatinel. “As with every refractive surgery procedure, the quality of communication with the patient, the precision of the biometry, and adequate IOL calculation are particularly important.”
Dr. Mertens agreed. “When doing multifocal IOL implantation, the preoperative diagnostic workup is essential. You must measure carefully, preferably with the IOLMaster, to personalize your outcome. Patients will not be satisfied when you are off target.”
The experts raised several additional points for consideration in patient and IOL selection.
Ask probing questions. Don’t presume that you know what your patients are thinking in terms of spectacle independence after IOL implantation, Dr. Gatinel cautioned. “In some cases, patients are not aware of the possibility offered by multifocal IOLs to become—or stay—spectacle free.” As a result, he said, “I believe that surgeons should probe the desire for spectacle independence at distance and near vision in every regular cataract patient.”
Dr. Daya agreed, adding that “in deciding which lens to choose, ascertaining patient requirements and lifestyle is vital.” For example, he said, “A patient came to see me and said he could not tell me what he did for a living, but he needed to jump out of aircraft and read maps in the dark. The only lens that could help him achieve reading in dim light conditions was the AT LISA. If I hadn’t learned this, I very likely would have chosen the FineVision lens because it has a better range of correction than that offered by the AT LISA, although intermediate vision is also respectable with the AT LISA.”
Beware of astigmatism. “Astigmatism is the enemy of multifocal implantation; it degrades the quality of each of the induced foci,” said Dr. Gatinel. “Corneal topography must be performed when a significant amount of corneal astigmatism is suspected. It will help to properly identify the preoperative axis of the corneal cylinder; in addition, it allows the physician to discover the presence of corneal conditions such as subclinical keratoconus or pellucid marginal degeneration, both of which represent contraindications to multifocal IOLs.” When regular corneal toricity is present and generates astigmatism, toric diffractive IOLs should be considered, he said.
Toric versions of the AT LISA and Lentis Mplus, as well as the AcrySof ReSTOR IQ, are available in Europe; but toric multifocals are not yet available in the United States.
Assess the tear film. Dr. Mertens recommends preoperative assessment of a patient’s tear film. “Both the amount and quality of tears are important; if either is deficient, patients will be unhappy after surgery.” When appropriate, he recommends that patients take supplemental vitamin B and omega-3 and omega-6 fatty acids one to two months before surgery.
Consider the type of cataract. “In my experience, patients who have significant amounts of lens opacity are better candidates [for multifocal IOLs] than those with relatively clear [crystalline] lenses,” said Dr. Gatinel. “The optical compromise incurred by multifocal IOLs will be subjectively less apparent, as the removal of an opaque crystalline lens and its replacement by a transparent pseudophakic lens will cause a dramatic increase in the quantity of light properly focused at the retinal plane, regardless of any energy split due to multifocality.”
Dr. Gatinel also said that “some forms of nuclear cataract cause myopic shifts that are sometimes well tolerated until the opacity significantly reduces BCVA. In my mind, these eyes, with a history of ‘natural multifocality,’ are potentially good candidates for multifocal IOL implantation.”
Coming to America?
It’s tempting—but ultimately unproductive—to speculate on when one or more of these IOLs might become available in the U.S. market. Nevertheless, cataract surgeons in the United States have been following overseas developments with keen interest.
Awaiting toric multifocals. “I’m looking forward to the first toric multifocal IOL in the United States,” said David F. Chang, MD, clinical professor of ophthalmology at the University of California, San Francisco, who also practices in Los Altos, Calif. “Diffractive multifocals are unforgiving of any astigmatism because any significant residual astigmatism reduces the image quality quite a bit. In patients with astigmatism, it can be very challenging to get good optical quality with a multifocal IOL by relying on astigmatic keratotomy, which introduces variables of individual response and regression. Having a toric diffractive multifocal available to us will be a very important step forward.”
Synchrony status. Mark Packer, MD, said, “If you ask me which IOL I’d love to have in my hands, I’d say the Synchrony [Visiogen/AMO]. And we spent years on that clinical trial.” Dr. Packer was principal investigator of the Synchrony IOL clinical trial in the United States, and he is clinical associate professor of ophthalmology at the Oregon Health & Science University in Portland, and practices in Eugene.
Although knowledgeable observers were expecting the Synchrony to become available during the first quarter of this year, the FDA did not approve the IOL. What now? Essentially, the manufacturer will have to generate more clinical data, said Dr. Chang, who was medical monitor of the U.S. Synchrony IOL clinical trial. “After discussions with the FDA, the company has decided to expand the U.S. study to specifically evaluate the newer design of the Synchrony.”
According to Dr. Chang, this model—which was developed as the company waited for FDA approval of the initial design—is expected to provide greater depth of field. “For that reason, AMO wants to eventually seek approval for the newer design.”
The Synchrony has been used in Europe since 2006. It has a movable plus-power anterior optic that is connected by spring haptics to a static compensatory minus- power posterior optic. The haptics allow movement of the front optic in response to changes in capsular tension and ciliary body tone.1
Bilateral implantation of the Synchrony provides the best results, said Dr. Chang. “You can assess how much accommodation you get after implanting the Synchrony in one eye; if you don’t get enough, you can do some mini-monovision for the second eye, without much sacrifice of binocularity.”
In a prospective study of 100 patients randomly assigned to receive either bilateral Synchrony or bilateral AcrySof ReSTOR (Alcon) IOLs, near and distance vision were equivalent with the two lenses. However, the Synchrony IOL provided better intermediate vision, and patients reported fewer problems with halos and glare.2
Dr. Chang said that the Synchrony is appropriate for patients who want greater spectacle independence and also want to avoid drawbacks associated with multifocal IOLs, notably halos, reduced contrast, and less intermediate-range vision. “In addition, an accommodating IOL can be offered to patients with slight maculopathy, such as a mild epiretinal membrane, who are not good multifocal IOL candidates,” he said.
___________________________
1 McLeod SD et al. J Cataract Refrac Surg. 2007;33(1):37-46.
2 Ossma IL et al. Functional Range of Vision After Binocular Implantation of the Multifocal or Dual-Optic Accommodating IOLs: Results of a Multicenter Prospective Trial. Presented at the ASCRS Symposium on Cataract, IOL and Refractive Surgery; April 5, 2009; San Francisco.
|
Novel Designs: Now and Future
Several novel IOLs that attempt to improve presbyopia through non-multifocal approaches have garnered a fair amount of interest among ophthalmologists abroad. For example, the Synchrony dual-optic accommodating IOL (Visiogen/AMO) is already available in Europe (see “Coming to America?” page 52).
Still other new designs are in the development phase. As with all investigational devices, there is a caveat: “Things can look good in the lab, but until you get solid clinical data, you just don’t know,” said Dr. Packer.
Following is a preview of some lenses that have not yet received either CE or FDA approval for marketing.
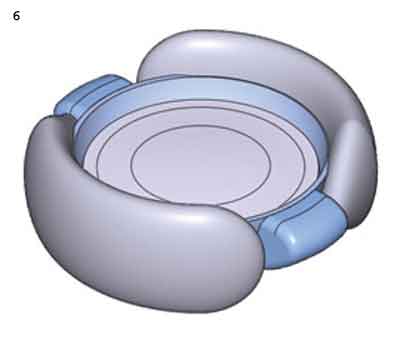
FluidVision. The soft haptics of the FluidVision IOL (PowerVision), which is placed in the capsular bag, serve as a storage area for silicone fluid. Accommodative effort forces the fluid to move into central fluid chambers, changing the shape of the anterior optic and shifting the eye’s focus. Early studies suggest that the lens can provide at least 5 D of accommodation.11
 |
|
FLUIDVISION. Fluid from the haptics changes the optic's shape.
|
Dynacurve. This sulcus-based accommodating IOL is similar to FluidVision in that it uses a gel or fluid to change the shape of the lens. But the Dynacurve (NuLens) is implanted in front of the collapsed capsular bag instead of inside it, and the capsular bag is used as a component of a dynamic diaphragm, which transfers forces as the ciliary muscles contract and relax.
The Dynacurve, which is fixed to the ciliary sulcus, consists of a small chamber filled with a silicone gel, a piston-like element, and a flexible membrane. When activated by the capsular diaphragm, the piston pressurizes the chamber and gel, which then modify the shape of the membrane.
In theory, the Dynacurve could provide up to 10 D of accommodation. In a pilot study of 10 patients with AMD and cataract, surgeons implanted the Dynacurve IOL in the eye with worse visual acuity. At the 12-month follow-up, the researchers found that both corrected and uncorrected near visual acuity in the treated eyes had improved, without compromising distance visual acuity.12
aKromalenses. The aKromalens SeeLens AC (Hanita) is neither a multifocal nor an accommodating IOL design. Rather, this aspheric, achromatizing diffractive lens, which fits through a 1.8-mm incision, was designed to improve image contrast and increase the depth of field by eliminating chromatic and spherical aberration. The manufacturer claims that the greater depth of field provides patients a large pseudo-accommodative range for functional activities.
In the first case series that evaluated the aKromalens, 26 patients received bilateral implants. Three-month results indicate that patients experienced an improvement of near vision in the range of 1.5 D to 2.0 D. The lens did not introduce intraocular aberrations, and patients reported no halos or glare. The researchers concluded that “the use of chromatic aberration correction increases depth of field in monofocal IOLs without inducing photic phenomena or decreasing contrast sensitivity function” and could prove to be a feasible alternative for presbyopia corrrection.13
___________________________
1 Alio JL et al. Ophthalmology. 2012;119(3):555-563.
2 Alfonso JF et al. J Cataract Refract Surg. 2007;33(11):1930-1935.
3 Fernandez-Vega L et al. Am J Ophthalmol. 2009;148(2):214-220.
4 Fernandez-Vega L et al. Eur J Ophthalmol. 2010;20(1):83-89.
5 Visser N et al. J Cataract Refract Surg. 2011;37(11):2034-2042.
6 Muñoz G et al. J Cataract Refract Surg. 2011;37(11):2043-2052.
7 Santhiago MR et al. J Cataract Refract Surg. 2012;38(2):215-220.
8 Alfonso JF et al. J Cataract Refract Surg. 2010;36(5):733-739.
9 Hoffer KJ. Personal History in Bifocal Intraocular Lenses. In: Maxwell A, Nordan LT, eds. Current Concepts of Multifocal Intraocular Lenses. Thorofare, N.J.: Slack, Inc; 1991:127-132.
10 Gatinel D et al. J Cataract Refract Surg. 2011;37(11):2060-2067.
11 Roux P. Early Clinical Results With PowerVision’s FluidVision IOL. Presented at the ASCRS Symposium on Cataract, IOL and Refractive Surgery; April 6, 2008; Chicago.
12 Alio JL et al. J Cataract Refract Surg. 2009;35(10):1671-1678.
13 Alio JL et al. Outcomes of a New Monofocal Achromatic Presbyopic Pseudophakic IOL: A Pilot Study. Presented at the American Academy of Ophthalmology Refractive Surgery Subspecialty Day; Oct. 21-22, 2011; Orlando, Fla.
Meet the Experts
DAVID F. CHANG, MD Clinical professor of ophthalmology at the University of California, San Francisco, and in practice in Los Altos, Calif. Financial disclosure: Was medical monitor of the Synchrony IOL trial in the United States and is a consultant for Alcon and AMO.
SHERAZ M. DAYA, MD, FACP, FRCS(Ed), FRCOphth Consultant and medical director of the Centre for Sight in East Grinstead, United Kingdom. Financial disclosure: Is a consultant to Carl Zeiss Meditec and PhysIOL.
DAMIEN GATINEL, MD Assistant professor and head of anterior segment and refractive surgery at the Rothschild Ophthalmology Foundation in Paris. Financial disclosure: Is a consultant to and has a patent ownership with PhysIOL.
KENNETH J. HOFFER, MD Clinical professor of ophthalmology, University of California, Los Angeles, and in practice at St. Mary’s Eye Center, Santa Monica, Calif. Financial disclosure: None.
ERIK L. MERTENS, MD, FEBOphth Medical director and eye surgeon at Medipolis in Antwerp, Belgium. Financial disclosure: None.
MARK PACKER, MD Clinical associate professor of ophthalmology at Oregon Health & Science University, Portland, and in practice in Eugene, Ore. Financial disclosure: Was principal investigator of the Synchrony IOL clinical trial in the United States and is a consultant to AMO.
|