By Sri Meghana Konda, MBBS, Randy C. Bowen, MD, MS, Jonathan S. Chang, MD, and Mihai Mititelu, MD, MPH
Edited By: Ingrid U. Scott, MD, MPH, and Bennie H. Jeng, MD
Download PDF
Familial exudative vitreoretinopathy (FEVR) is an inherited vitreoretinal disorder characterized by incomplete or anomalous vascularization of the peripheral retina. The avascular peripheral retina leads to various degrees of retinal ischemia, which can cause neovascularization, vascular dragging, radial retinal folds, retinal exudates, vitreous hemorrhages, and tractional retinal detachments (RDs). About half of FEVR cases are associated with known genetic mutations, and the etiology is unknown in the remainder.1
This disease entity was first described by Chriswick and Schepens in 1969, in a report of six patients from two families who had fundus findings similar to those observed in retinopathy of prematurity (ROP). In a recent multicenter investigation of 199,851 newborns who received screening ocular exams, the reported incidence of FEVR was 0.11% (219 cases).2 The prevalence of FEVR is likely to be underestimated; vascular abnormalities in the peripheral retina may be overlooked on routine exams, and widefield fluorescein angiography (FA) is not routinely used in clinical practice.3
Presentation. The presentation and severity of the disease are highly variable, even among family members and sometimes between the two eyes of the same individual. Although avascular peripheral retina may be the only manifestation, some cases may advance to neovascularization, exudation, sub- or intraretinal hemorrhage, hyaloid contraction, and RD.4 Because of the variability of expression and incomplete genetic penetrance, the disease is often misdiagnosed. It is important to distinguish FEVR from other conditions with similar findings, including Norrie disease (ND), ROP, RD, Coats disease, retrolental fibroplasia, sickle cell retinopathy, and Eales disease (see “Differential Diagnosis”). Widefield FA remains the most helpful diagnostic tool.3
Pathophysiology
Incomplete vascularization of peripheral retina in FEVR is the result of developmental abnormalities.
In normal development, vasculogenesis begins when spindle-shaped mesenchymal precursor cells, migrating to the retina through the optic disc, differentiate into endothelial cells, which aggregate to form patent vessels that expand centrifugally. Next, angiogenesis occurs when sprouts of blood vessels emanate from the preexisting vascular framework, increasing the vascular density of the immature plexus and extending it peripherally. This process is mediated by vascular endothelial growth factor (VEGF) expressed by astrocytes in response to localized retinal hypoxia, which highlights the importance of VEGF in ocular vascularization.5
Most genes associated with FEVR affect pathways regulating the development of the secondary embryonic retinal vasculature via angiogenesis. Thus, the gene mutations in FEVR lead to incomplete or anomalous vascularization of the peripheral retina. In normal development, hyaloid vasculature forms and then regresses prior to retinal vascular development, but persistent hyaloid vasculature due to failure of regression is sometimes seen in FEVR.
Genetics
Approximately 50% of FEVR cases have a genetic cause, and the disease can be transmitted through autosomal dominant, autosomal recessive, or X-linked patterns of inheritance. FEVR is associated with various gene mutations. Autosomal dominant FEVR, the most common inherited type, is associated with mutations in the frizzled-4 (FZD4), low-density lipoprotein receptor–related protein 5 (LRP5), tetraspanin-12 (TSPAN12), kinesin family member 11 (KIF11), and zinc finger protein 408 (ZNF408) genes.
X-linked FEVR and ND are associated with mutations in the NDP gene, which produces the norrin protein. The Wnt canonical pathway plays a central role in cell proliferation, differentiation, and migration in the body, including formation of retinal vasculature, by promoting accumulation of cytosolic b-catenin. Consequently, gene mutations that encode the Wnt receptor FZD4, coreceptor LRP5, or the ligand norrin result in retinal vascular disruption.6
Diagnosis
Widefield FA is the gold standard for diagnosing FEVR.3 In pediatric patients, evaluation may require examination and imaging under anesthesia. Optical coherence tomography angiography has demonstrated parafoveal microvascular defects with vascular density correlating to vision loss in FEVR without RDs.7
Screening asymptomatic family members with widefield FA may aid in early detection and, if needed, treatment of FEVR to prevent progression and vision loss. It also facilitates genetic counseling for individuals of childbearing age.
Clinical features. The progression and phenotypic expression of the disease vary among individuals.1 As previously noted, there may even be asymmetric clinical findings in the fellow eye of the same patient. Pediatric patients often have a more progressive disease course than adults, which can lead to serious, vision-threatening complications such as RD. Adults typically have a more indolent course. However, in both adults and children, progression can commence or halt at any time during the course of the disease.
Mild disease. Peripheral avascular retina can be seen vertically or in a wedge or V shape, with the apex pointing toward the macula. The peripheral avascular retina initially presents without neovascularization but can develop neovascularization or arteriovenous fistulas at the edge of the avascular zone.4 Hyaloid remnants may be seen. Patients are usually asymptomatic at this stage and are diagnosed incidentally on fundus examination with an indirect ophthalmoscope or FA.5
Moderate disease. Neovascularization leads to fibrous changes that can manifest as strong vitreoretinal adhesions and/or fibrovascular stalks. These fibrovascular stalks and/or vitreoretinal bands place traction on the posterior pole, pulling the macula toward the origin of the neovascularization. The traction can cause ectopic macula and may distort the optic disc, significantly affecting vision. Continued fibrovascular activity may lead to RD outside the macula, which can further drag on the posterior pole. There may be evidence of shallow intraretinal or subretinal exudation.1
Severe disease. Severe extraretinal neovascularization may result in subtotal macular or total rhegmatogenous and/or tractional retinal detachments. In a study of 273 eyes, 64% of FEVR patients developed RDs.1 Some patients with severe tractional peripheral displacement of the retina develop a falciform retinal fold that originates from the optic disc and stretches across the macula. Ranchod et al.1 reported that the majority of retinal folds were radial and were seen in every quadrant, not just temporally, as was previously believed. Radial folds are classified as dry, knifelike, and broad, and they may be associated with subretinal exudate or blood. In advanced cases, anterior segment complications including cataract, neovascular glaucoma, and band keratopathy may be present.1,5
Differential Diagnosis
The fundus findings in FEVR are similar to those of several other diseases. Some pointers for differentiating among them are included below.
Retinopathy of prematurity. FEVR and ROP are phenotypically similar, as patients have an area of avascular retina bilaterally and leaky peripheral retinal blood vessels. FEVR and ROP also have some genetic similarities: Four variants of three FEVR genes (FZD4, LRP5, and TSPAN12) have recently been associated with ROP.8
Despite similar clinical features, FEVR is classically distinguished from ROP according to whether the patient was born full term or prematurely. Further, ROP generally progresses on a predictable timeline, with retinal neovascularization occurring at approximately 37 weeks postmenstrual age followed by RD around 41 weeks. In contrast, FEVR is more unpredictable and may remain dormant throughout a patient’s life or progress in childhood or adulthood.1
Norrie disease. ND is associated with X-linked mutations of NDP and presents with ocular manifestations similar to those noted in FEVR. However, ND usually has coexisting systemic conditions of progressive deafness and cognitive delay.
There is also a difference in the location of mutations for the norrin protein, a product of the NDP gene. Mutations in the cysteine residues of the norrin protein lead to ND, while mutations in the noncysteine residues lead to X-linked FEVR.
Coats disease. RDs with significant exudations in Coats disease can resemble FEVR. However, Coats is unilateral and sporadic, and it primarily affects boys, with diagnosis typically between age 8 and 16 years.
Persistent fetal vasculature (PFV). Although failure of regression of hyaloid vessels is seen in both FEVR and PFV, in the latter condition it is usually unilateral and not inherited.
Eales disease. Although they are initially asymptomatic, Eales patients typically present with periphlebitis and obliterative retinal vasculopathy affecting the peripheral retina, which can lead to neovascularization and tractional RDs.
Sickle cell retinopathy. Findings of sickle cell retinopathy include peripheral neovascular “sea-fans” and salmon patches, which can lead to tractional RDs. Systemic and laboratory workup can further differentiate this disease from FEVR.
Toxocariasis. This ocular roundworm infection forms a granuloma with a stalk that can be mistaken for a fibrovascular stalk wrapped in retinal folds found in FEVR. However, toxocariasis also has a significant component of posterior uveitis, which is absent in FEVR.
Osteoporosis pseudoglioma syndrome. This syndrome is characterized by reduced bone mineralization resulting in increased risk of bone fractures in childhood, along with an ocular FEVR-like presentation. Cognitive decline and muscle hypotonia may also be present.
Incontinentia pigmenti (IP). An X-linked ectodermal dysplasia, IP usually presents with characteristic dermatologic changes but may have retinal findings similar to FEVR. Ophthalmic manifestations are present in about one-third of cases.
Staging
Various grading systems to classify FEVR have been proposed. Initially, staging was based on clinical findings but has undergone revisions to incorporate FA characteristics and other developments.9 Table 1 outlines a commonly used staging system.1
Table 1: Staging of FEVR
| Stage |
Description |
| 1 |
Avascular peripheral retina |
| 2 |
Retinal neovascularization
A. Without exudate
B. With exudate |
| 3 |
Extramacular retinal detachment
A. Without exudate
B. With exudate |
| 4 |
Macula-involving retinal detachment, subtotal
A. Without exudate
B. With exudate |
| 5 |
Total retinal detachment |
| SOURCE: Ranchod TM et al. Ophthalmology. 2011;118(10):2070-2075. |
Management
Management of FEVR depends on the stage and severity of retinal findings. Stage 1 can be managed with observation for signs of neovascularization. In stages 2 to 5, the goal is to prevent the progression and sequelae of neovascularization. Laser photocoagulation and/or cryotherapy to address neovascularization are indicated in stage 2. Given that VEGF affects the Wnt/β-catenin pathway, anti-VEGF therapy may decrease hemorrhage and exudation10; however, this may worsen the vitreoretinal traction owing to contraction of fibrovascular tissue.
Continued fibrovascular proliferation can lead to rhegmatogenous or tractional RDs requiring surgery. Although surgical intervention is recommended for the complications of FEVR, the prognosis for improved visual acuity (VA) is guarded depending of the level of severity. In a recent study, 22 patients ages 3-6 with stage 5 FEVR underwent either lens-sparing vitrectomy, vitrectomy with lensectomy, or lensectomy alone. Final VA was tested on 17 eyes, which ranged from no light perception (NLP) to 30/200.11 Surgical techniques include pars plana vitrectomy, scleral buckling, or a combination of both with or without lensectomy.
Because of the unpredictable disease course, risk of reactivation, and chronic nature of FEVR, lifelong monitoring with twice-yearly exams (as well as widefield FA in eyes at risk) is indicated, and treatment may be needed throughout the patient’s life.
Conclusions
FEVR is a complex retinal condition that is often underrecognized and can lead to severe vision loss. Early recognition, with careful bilateral dilated exams and widefield FA, is important in making the correct diagnosis. Once there is evidence of neovascularization, laser photocoagulation treatment of the peripheral avascular retina should be initiated. Prompt treatment is aimed at preserving vision and preventing sight-threatening complications of neovascularization, including vitreous hemorrhage, RD, and neovascular glaucoma.
___________________________
1 Ranchod TM et al. Ophthalmology. 2011;118(10):2070-2075.
2 Tang H et al. Br J Ophthalmol. 2018;102(12):1742-1746.
3 Kashani AH et al. Ophthalmology. 2014;121(1):262-268.
4 Miyakubo H et al. Ophthalmology. 1984;91(12):1524-1530.
5 Gilmour DF. Eye (Lond). 2015;29(1):1-14.
6 Junge HJ et al. Cell. 2009;139(2):299-311.
7 Zhang J et al. Retina. Published online March 6, 2019.
8 Mohd Khair SZN et al. J Ophthalmic Vis Res. 2019;14(2):171-178.
9 Pendergast SD, Trese MT. Ophthalmology. 1998;105(6):1015-1023.
10 Lu YZ et al. Int J Ophthalmol. 2018;11(6):976-980.
11 Fei P et al. Retina. 2016;36(8):1480-1485.
___________________________
Dr. Konda is a research fellow, Dr. Bowen is an ophthalmology resident, Dr. Chang is an assistant professor, and Dr. Mititelu is an associate professor; all are in the Department of Ophthalmology and Visual Sciences at the University of Wisconsin School of Medicine and Public Health in Madison. Financial disclosures: None.
Case Reports
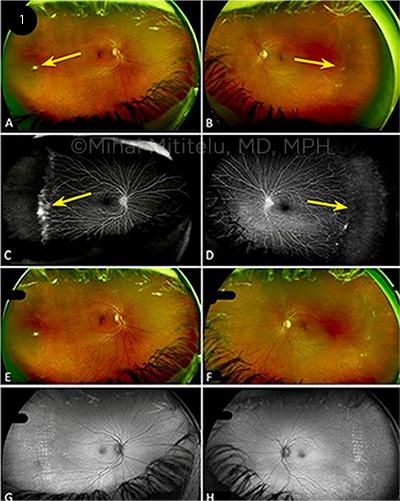
CASE 1. A 56-year-old systemically healthy woman was initially diagnosed with stage 1 FEVR by an outside provider. Because of a change in her insurance, she presented to our clinic five years later, at age 61, for follow-up. She reported no photopsia, floaters, scotomas, or reduced vision. She had been born full term, without complications. VA was 20/25 in each eye. On our examination, the fundus exam showed lack of vascularization in the temporal retina in both eyes, with a white fibrovascular ridge (Figs. 1A, 1B, arrows). FA revealed bilateral peripheral nonperfusion in the temporal quadrants, with leakage from retinal neovascularization (Figs. 1C, 1D, arrows), which demonstrated progression of disease to stage 2 FEVR.
Because the neovascularization had progressed since the prior presentation, laser photocoagulation was performed in the temporal ischemic region in both eyes. The patient responded well to laser therapy; she had no progression of neovascularization or ischemia and retained excellent visual acuity (Figs. 1E-1H).
This case illustrates the importance of close monitoring for development of peripheral neovascularization in patients with early-stage FEVR. Timely peripheral laser treatment can prevent tractional retinal detachments.
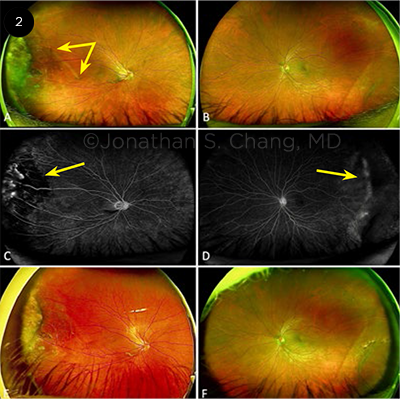
CASE 2. A 41-year-old man with an unremarkable medical history presented for evaluation of a raised, pigmented peripheral retinal lesion. The patient was asymptomatic, with no family history of retinal disease or history of prematurity. He reported no photopsia or shadows in vision. VA was 20/40 and 20/25 in the right and left eyes, respectively. The fundus exam of the right eye showed a dragged macula (Fig. 2A, double-headed arrow) with temporal fibrovascular tissue. The left eye (Fig. 2B) had a normal fovea and infratemporal retinoschisis. In the right eye, FA demonstrated a V-shaped area of avascular/limited perfusion in the temporal retina (Fig. 2C, arrow), with mild leakage. In the left eye, FA showed mild nonperfusion in the far temporal periphery (Fig. 2D, arrow). After the patient received laser photocoagulation therapy to right eye, he had no further complications, no increase in fibrosis, and no new areas of neovascularization (Figs. 2E, 2F).
|