By Linda Roach, Contributing Writer, interviewing M. Elizabeth Hartnett, MD, Amy K. Hutchinson, MD, and Stephen J. Kim, MD
Download PDF
In a major shift for pediatric ophthalmic care, drugs to inhibit aberrant intraocular angiogenesis have largely supplanted laser photocoagulation as first-line treatment for the most severe cases of retinopathy of prematurity (ROP).
“This is done fairly commonly now by many practitioners in the United States and throughout the world. It is becoming increasingly recognized and accepted, because it enables us to preserve the retina in children with very advanced zone 1 ROP or aggressive posterior disease,” said Stephen J. Kim, MD, at Vanderbilt University in Nashville, Tennessee. “In the past, if you lasered these eyes at this stage, you would destroy much of their peripheral vision.”
 |
|
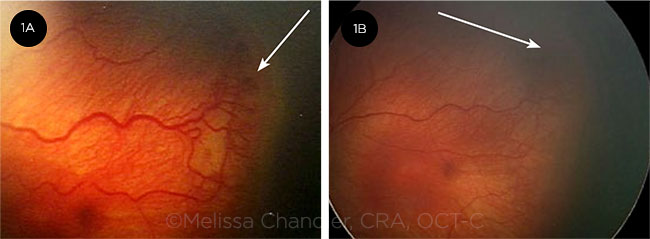
BEFORE AND AFTER. In this case of stage 3 ROP, dilated tortuous vessels (plus disease) are evident before anti-VEGF treatment (1A). One week later, there is less tortuosity, reduced stage 3 ROP, and regrowth of physiologic vascularization (1B).
|
Where We Are Now
Guidance on how and when to use anti-VEGF medications in ROP patients has emerged over the past several years from a few prospective clinical studies and some clinical trials comparing drug and laser treatment. (There also is an ongoing prospective, phase 1 dose de-escalation study sponsored by the Pediatric Eye Disease Investigator Group [PEDIG] and the NEI.1) And while most of the studies have investigated the use of bevacizumab (Avastin), attention to ranibizumab (Lucentis) has begun to rise.
Guidance statements. In 2017, the Academy’s Ophthalmic Technology Assessment Committee (OTAC) reported finding Level II and III evidence in the literature that intravitreal therapy to inhibit VEGF is at least as effective as laser photocoagulation for achieving regression of acute ROP.2
And in an updated policy statement published in December, the American Academy of Pediatrics prominently included intravitreal anti-VEGF therapy among the recommendations for managing some types of ROP.3 The statement was developed with representatives from the Academy, the American Association for Pediatric Ophthalmology and Strabismus, and the American Association of Certified Orthoptists.
Fear of systemic problems. The onset of off-label usage of VEGF inhibitors sparked concerns that circulation of the drugs elsewhere in the body might reduce systemic VEGF sufficiently to hamper organ development, and these concerns are still being debated today, said Amy K. Hutchinson, MD, at Emory University in Atlanta.
“There’s a lot of controversy at the heart of this topic,” Dr. Hutchinson said. “Some physicians are reluctant to use bevacizumab until more is known about its effects outside the eye on developing organs like the brain, kidney, and lungs. In addition, we are still studying bevacizumab to determine the smallest effective dosage to minimize such risks.”
Residence time in the body. M. Elizabeth Hartnett, MD, at the University of Utah’s John A. Moran Eye Center in Salt Lake City, said that the full-length antibody bevacizumab inactivates multiple VEGF isoforms and persists in the body for weeks. But, as a smaller antibody fragment, ranibizumab blocks fewer VEGF receptors and disappears from circulation faster, she noted.
“If you inject bevacizumab into the eye, it gets into the circulation and can be detected for several months after a single dose,” she said. In contrast, preliminary data from a large, multicenter trial showed that serum VEGF levels in infants treated with ranibizumab returned to baseline within two weeks after a single intravitreal treatment,4 she said. “Nonetheless, the studies suggest that more than one treatment with ranibizumab may be needed, and the repetition of use may extend the inhibition longer than the two weeks,” she said.
“We need more information before advocating either drug for all types of treatment-warranted ROP. However, the evidence for zone 1 treatment-warranted ROP seems to favor consideration for anti-VEGF treatment,” Dr. Hartnett said.
Variations in clinician preference. It is too early to know how the speed of anti-VEGF drug clearance from the body might affect safety or long-term outcomes of therapy, but on the premise that less systemic exposure is better, Dr. Kim prefers ranibizumab for treating ROP. “At Vanderbilt we have moved almost entirely to ranibizumab, and we generally avoid bevacizumab. The reasons are theoretical at this time but are based on ranibizumab’s faster clearance and reduced chance for systemic inhibition of VEGF,” he said.
In contrast, Drs. Hartnett and Hutchinson said they tend to use bevacizumab for zone 1 treatment-warranted ROP at a dose of 0.25 mg, or at lower doses as part of the PEDIG de-escalating dose study, for two reasons: 1) The literature on bevacizumab’s effectiveness and apparent safety is deeper than it is for ranibizumab, and 2) bevacizumab is both cost-effective and widely available around the world.
A Look at Early Outcomes
Resolution and recurrence. Results from clinical trials have shown that a single intravitreal injection of either medication successfully resolved ROP in many eyes. But bevacizumab’s greater potency compared with ranibizumab appears to result in fewer cases that required late retreatment for recurrent disease by six months postinjection.
For instance, in the RAINBOW trial (a randomized trial comparing low-dose ranibizumab with laser), preliminary analysis found that 31% of the ranibizumab infants had recurrent ROP requiring retreatment in the six months after the initial injection, Dr. Hartnett said.4 That compares to a 23% retreatment rate at the same time point in children treated with bevacizumab during the ROP1 study.5
In addition, a study in 241 infants treated with bevacizumab found late reactivation of proliferative disease in 8.3% of the children, and retreatments had to be performed as long as 65 age-adjusted weeks after initial treatment.6
Anti-VEGF plus laser. “Given the risk of late recurrence of ROP with ranibizumab and loss to follow-up, we have a policy at Vanderbilt of performing laser treatment to avascular retina in all ranibizumab-treated eyes after normal retinal vascularization has ceased” and before the infants are discharged, Dr. Kim said.
More normal structure? Anti-VEGF therapy in eyes with zone 1 (the most posterior) ROP has a potential advantage over laser photocoagulation: the possibility that it can nondestructively enable healthy intraretinal blood vessels to mature and extend a bit across formerly avascular retina, Dr. Hartnett said. “Anti-VEGF offers an ability to extend normal retinal vascularization into zone 2 in some eyes. I think that’s exciting,” Dr. Hartnett said. Some case studies also suggest that anti-VEGF-treated eyes may be less myopic.7
Risk of avascular retina. Investigators in the CARE-ROP study have reported a high incidence of avascular retina in ranibizumab-treated eyes, Dr. Hartnett said. The rate was 84% in the higher-dose eyes, compared to 18% of eyes treated with bevacizumab in the ROP1 study.
“We can’t directly compare these trials, of course, since they are not head-to-head studies and they had different enrollment criteria, [evaluated] different zones of disease, were from different regions of the world, and had different outcomes, but we can make observations about them.” Nonetheless, she said, “We don’t know what the observations mean in the long term.” For instance, she said, “The avascular retina could stimulate VEGF and cause recurrent ROP. There also have been some reports of recurrent retinal detachment even up to 2.5 years after a single anti-VEGF treatment.”
Difficulties evaluating developmental delays. To alleviate concerns about potential damage to the brain and other organs from systemic anti-VEGF exposure, researchers must find ways to tease out any VEGF-related anomalies from the natural history of prematurity, Dr. Hutchinson said.
“A handful of studies have been published with conflicting conclusions about whether bevacizumab is associated with poorer neurodevelopmental outcomes than laser. Since patients in these studies were not randomized, there is a strong potential for bias,” she said.
Are Outcomes Improved?
The question of whether anti-VEGF therapy improves outcomes takes clinicians into unknown territory. Pediatric ophthalmologists are hoping that, as treated children enter their school years, normalized retinal structure will translate into better visual functioning than if they had been treated with laser monotherapy. But, like so much else in the anti-VEGF treatment field, this possibility remains to be demonstrated scientifically. “This is all anecdotal and theoretical,” Dr. Kim said. “We don’t know what will happen in five years or beyond with these children.”
Dr. Hutchinson concurred. “I think most of us would agree that the published literature suggests that for zone 1 ROP, anatomical outcomes, recurrence rates, and rates of high myopia are better with bevacizumab than with laser. However, since we have not yet carefully studied retinal function in bevacizumab-treated eyes, we cannot say for certain that bevacizumab is the best treatment for zone 1 ROP,” she said.
One of the first infants Dr. Hutchinson treated with bevacizumab is now 7 years old and appears to have overcome early developmental delays, she said. “He performed poorly on the Bayley infant skill and development test at age 2 and was labeled at having ‘severe impairment,’ but he is now excelling in school.”
To progress beyond the anecdotal, it would be helpful if future anti-VEGF trials for ROP included objective tests of retinal function, such as visual field and electrophysiological testing, Dr. Hutchinson said. “On the other hand, the longer we go without seeing obvious differences in the health and development in our formerly premature patients treated with bevacizumab, the more comfortable we start to feel,” she said. But—as she acknowledged—“that might be a false sense of security.”
___________________________
1 www.ClinicalTrials.gov Identifier: NCT02390531. Accessed Jan. 7, 2019.
2 VanderVeen DK et al. Ophthalmology. 2017;124(5):619-633.
3 Fierson WM et al. Pediatrics. 2018;142(6). http://pediatrics.aappublications.org/content/142/6/e20183061. Accessed Jan. 9, 2019.
4 Hartnett ME. “Anti-VEGF treatment for ROP: Clinical trials and phenotypic differences worldwide.” Presented at: Retina Subspecialty Day; Oct. 26, 2018; Chicago.
5 Wallace DK et al. JAMA Ophthalmol. 2017;135(6):654-656.
6 Mintz-Hittner HA et al. Ophthalmology. 2016;123(9):1845-1855.
7 Mintz-Hittner HA, Geloneck MM. Eye Brain. 2016;8:135-140.
___________________________
Dr. Hartnett holds the Calvin S. and JeNeal N. Hatch Presidential Endowed Chair in Ophthalmology and Visual Sciences at the University of Utah in Salt Lake City. She is professor of ophthalmology in the Vitreoretinal Medical and Surgical Service; director of pediatric retina; and principal investigator in the Retinal Angiogenesis Laboratory at the university’s John A. Moran Eye Center. She also is adjunct professor of pediatrics and adjunct professor of neurobiology and anatomy at the university. Relevant financial disclosures: Novartis: S; Parexel: S.
Dr. Hutchinson is professor of ophthalmology and interim director of the Section of Pediatric Ophthalmology and Strabismus at Emory University in Atlanta. She also is an ex-officio member of the Academy’s OTAC Pediatrics/Strabismus Panel. Relevant financial disclosures: None.
Dr. Kim is professor of ophthalmology and visual sciences, director of the ophthalmology fellowship program, and director of the Retina Division at Vanderbilt University in Nashville, Tenn. He also is chairman of the Academy’s OTAC. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Hartnett Knights Templar Eye Foundation: C; Lippincott Williams and Wilkins: P; Novartis: S; Parexel: S.
Dr. Hutchinson Welch Allyn: C.
Dr. Kim None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|