By Nathaniel Gelinas, DO, Alan G. Palestine, MD, and Paula E. Pecen, MD
Edited By: Ingrid U. Scott, MD, MPH
Download PDF
Richard Ranger* was referred to our uveitis clinic for worsening intraocular inflammation in his left eye. Three months prior, the 73-year-old had undergone uncomplicated phacoemulsification with placement of a toric IOL in his left eye.
Postcataract problems. One month postoperatively, his primary cataract surgeon noted worsening visual acuity (VA) and elevated intraocular pressure (IOP), as well as early posterior capsular opacification (PCO), in the surgical eye. The surgeon discontinued Mr. Ranger’s difluprednate 0.05% drops, which had been prescribed for post-op care, and started him on loteprednol 0.5% drops, twice daily.
Nd:YAG capsulotomy, and further problems. One week later, Mr. Ranger returned for an uncomplicated Nd:YAG capsulotomy. One month after that, he returned, complaining of continued decreased vision and worsening photophobia in the left eye during that month. The slit-lamp exam revealed 2+ anterior chamber cells and 2+ anterior vitreous cells in the left eye. Mr. Ranger was started on difluprednate 0.05% twice daily and brimonidine/timolol 0.2%/0.5% twice daily in the left eye and referred to our uveitis service.
We Get a Look
Mr. Ranger presented to our uveitis clinic two weeks later.
Medical history. Mr. Ranger’s medical history was significant for atrial fibrillation, hypertension, and a cerebral vascular accident (CVA). We noted that he was taking rivaroxaban (Xarelto) for the atrial fibrillation.
Ocular history. His ocular history was significant for nonexudative age-related macular degeneration (AMD) in the right eye and exudative AMD in the left eye.
Exam. Mr. Ranger’s VA was 20/25 in his right eye and 20/125 in the left. His pupils were equally round and reactive with no relative afferent pupillary defect. Confrontation visual fields, IOPs, and extraocular movements were all within normal limits.
The anterior segment exam of the right eye was unremarkable other than a 2+ nuclear sclerotic cataract with a small central posterior subcapsular cataract. The anterior segment exam of the left eye was significant for trace conjunctival injection, 3+ anterior chamber cells, a few round white opacities on the posterior aspect of the IOL, and a white capsular infiltrate noted temporally (Fig. 1).
The posterior segment exam of the right eye was unremarkable other than a posterior vitreous detachment and drusen. The posterior segment of the left eye had 2+ anterior vitreous cells with moderate posterior vitreous haze, a posterior vitreous detachment, a blunted foveal reflex, and an involuted/inactive nasal choroidal neovascular membrane (CNVM) with drusen. There was no retinal whitening or vascular sheathing and no chorioretinal lesions.
We started Mr. Ranger on prednisone 40 mg orally, and the difluprednate 0.05% was increased to three times daily.
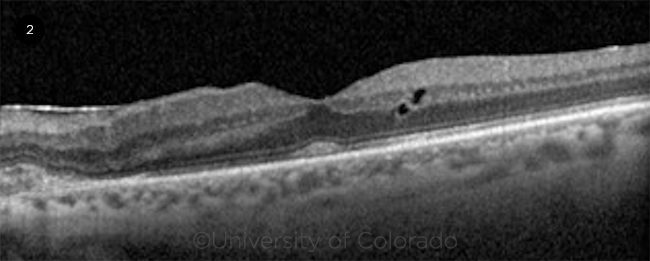
Testing. Optical coherence tomography (OCT) of the right eye was unremarkable, except for a few drusen. OCT of the left eye (Fig. 2) showed mild intraretinal fluid temporal to the fovea, drusen, and a mild epiretinal membrane with a nasal inactive CNVM.
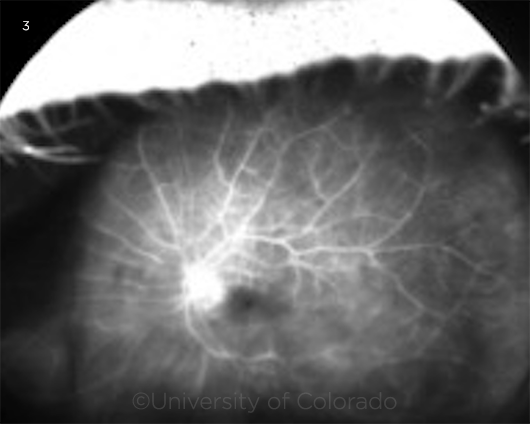
Ultra-widefield fluorescein angiography (UWF-FA) of the right eye was unremarkable. UWF-FA of the left eye (Fig. 3) showed optic disc leakage, diffuse vessel leakage, and foveal leakage in a petaloid pattern consistent with cystoid macular edema.
 |
|
AT THE SLIT LAMP. In the patient’s left eye, several white opacities on the posterior aspect of the IOL are seen with wide angled broad beam (1A) and transillumination (1B).
|
Differential Diagnosis
The patient presented with a unilateral panuveitis that occurred approximately three months after cataract surgery and that worsened shortly following an Nd:YAG capsulotomy.
Endophthalmitis? Given the recent cataract surgery and the worsening after the Nd:YAG capsulotomy, the possibility of an indolent, subacute endophthalmitis was considered, most likely caused by the bacteria Propionibacterium acnes (P. acnes). However, other organisms—including fungi and gram-negative bacteria—are known to lead to a similar presentation.
A previously undiagnosed condition? The possibility of a previously undiagnosed and now flared chronic inflammatory or infectious condition such as sarcoidosis, syphilis, or tuberculosis was also included in the differential diagnosis.
Masquerade syndrome? Lastly, the possibility of a “masquerade syndrome,” such as primary intraocular lymphoma, was considered.
 |
|
OCT OF THE LEFT EYE. Imaging reveals intraretinal fluid temporal to the fovea, drusen, an inactive choroidal neovascular membrane nasal to the fovea, and a mild epiretinal membrane.
|
Workup
Laboratory testing and x-ray. An anterior chamber paracentesis was performed and sent for aerobic and anaerobic cultures, as well as viral polymerase chain reaction (PCR), but with no positive results. A complete blood count (CBC), comprehensive metabolic panel (CMP), syphilis IgG, QuantiFERON-TB Gold, angiotensin converting enzyme (ACE), and chest x-ray were all within normal limits.
Further testing. Two weeks later, the patient returned for a follow-up visit and was noted to have mild improvement; however, persistent inflammation was still evident.
Given that there had not been a significant improvement, we decided to proceed with a pars plana vitrectomy (PPV)/vitreous biopsy combined with sub-Tenon injection of triamcinolone acetonide. Vitreous IL-10 and IL-6 ratios were also obtained.
Making the Diagnosis
Anaerobic culture results from the vitreous biopsy demonstrated gram-positive pleomorphic rods in clusters consistent with P. acnes. The remaining cultures demonstrated no growth. The IL-10/IL-6 ratio was reported as 0.5. Given the culture results, the diagnosis of P. acnes endophthalmitis was established. Mr. Ranger returned for intravitreal vancomycin with two repeat injections at one-week intervals. After three intravitreal vancomycin injections, the anterior chamber has remained quiet with no signs of recurrent inflammation—and Mr. Ranger is off all topical steroid drops.
 |
|
UWF-FA OF THE LEFT EYE. Late phase UWF-FA of the left eye shows optic disc leakage and diffuse vessel leakage.
|
Discussion
P. acnesis a gram-positive, pleomorphic bacillus that is part of the normal microbiota of the skin, oral cavity, and gastrointestinal tract.1
Taxonomy update. In 2016, based on genetic and biochemical phylogenetic studies, P. acnes was restructured in the new genus Cutibacterium and, thus, is correctly referred to as Cutibacterium acnes.2 However, for clarity and to conform with the general ophthalmologic literature, this case report will continue to use the term P. acnes.
Common associations. P. acnes is most commonly associated with the skin condition acne vulgaris but is also associated with postoperative infections, prostheses failure, and inflammation of lumbar nerve roots leading to sciatica.1
Virulence factors. P. acnes possesses numerous unique virulence factors that allow it to establish a chronic indolent infection including bacterial seeding, modification and manipulation of the host immune response, and biofilm formation.1
Role in endophthalmitis. P. acnes endophthalmitis was first described by Meisler et al. in 1986 as a chronic, indolent intraocular inflammation occurring after extracapsular cataract extraction.3 The organism thrives in the anaerobic environment between the lens capsule and the IOL. Although it prefers anaerobic conditions, the aerotolerant anaerobe can abide small amounts of oxygen. The hallmark feature of P. acnes endophthalmitis is white intracapsular plaques, which have been shown histologically to be sequestered organisms.4,5
Diagnostic challenges. Recognizing P. acnes endophthalmitis is challenging because early stages may resemble sterile postoperative inflammation. Classically, P. acnes produces a chronic, indolent, low-grade panuveitis that often shows an initial response to topical corticosteroids and frequently lacks the “classic” signs of endophthalmitis.4,5 Hypopyon, present in around 85% of cases of acute postoperative endophthalmitis, is only present in one-third of P. acnes endophthalmitis cases.4 Inflammation often worsens significantly following Nd:YAG capsulotomy, which is presumably due to release of previously sequestered organisms.6
Lab work. P. acnes is fastidious. It is essential to monitor samples longer than typical, as the average time to culture positivity is eight to 10 days and can be as long as 14 days.4,5 Samples can be obtained from either the aqueous or vitreous. Importantly, many other infectious organisms are known to cause a chronic postoperative endophthalmitis including coagulase-negative staphylococci, gram-negative organisms, and fungi, and this underlies the importance of obtaining aerobic, anaerobic, and fungal cultures.5
Treatment. The treatment of P. acnes endophthalmitis can be challenging, and no clear standards of care exist. Treatment options include:
- intraocular antibiotics alone (vancomycin +/- amikacin or clindamycin)
- PPV plus intraocular antibiotics
- PPV, partial capsulectomy, and intraocular antibiotics
- PPV, total capsulectomy, IOL removal or exchange, and intraocular antibiotics.4,5
Recurrence rates. In 1999, Aldave et al. published a retrospective review of culture-positive P. acnes endophthalmitis patients. The authors found a 93% recurrence rate for patients treated with intraocular antibiotics alone, while PPV along with intraocular antibiotics showed only a 50% recurrence rate.5 The recurrence rate for treatment with PPV, partial capsulectomy, and intraocular antibiotics was 23%, while PPV, total capsulectomy, IOL removal or exchange, and intraocular antibiotics was 0%.3 The authors did not find a relationship between the degree, severity, or chronicity of intraocular inflammation and recurrence rates.5
Also in 1999, Clark et al. published a similar retrospective review and reported similar success rates with each treatment regimen. Again, no patients in the PPV, total capsulectomy, IOL removal or exchange, and intraocular antibiotics group showed recurrences. Importantly, the researchers reported significantly longer durations of inflammation with more conservative initial management but no significant difference in final VA among the groups.4
Drug delivery. In contrast to techniques generally employed for intravitreal drug delivery, injection of intravitreal antibiotics directly into or adjacent to the capsular bag can be considered. Other authors have suggested a “two-compartment” approach whereby antibiotics are injected simultaneously into the aqueous and vitreous.
Management. In most cases of P. acnes endophthalmitis, it is reasonable to pursue conservative management with no surgery or limited surgery as initial therapy. In the era of refractive cataract surgery, with high patient expectations, the prospect of potentially avoiding IOL explantation and anterior chamber IOL placement is appealing. The complications of anterior chamber IOLs are well documented. It is important to discuss with each patient the high risk of recurrence with more conservative therapies. A tailored approach to each patient, after a comprehensive discussion of the risks and benefits of each treatment option, is essential.
Conclusions
P. acnes endophthalmitis is an uncommon, but important, cause of chronic, indolent post-op inflammation. Characteristic features include the presence of white intracapsular plaques with chronic, low-grade vitritis. Inflammation often shows a partial response to topical corticosteroids and frequently worsens after an Nd:YAG capsulotomy.
___________________________
*The patient’s name is fictitious.
___________________________
1 Bhatia A et al. Propionibacteriuma acnes and chronic diseases. In: Knobler SL et al., eds. The Infectious Etiology of Chronic Diseases: Defining the Relationship, Enhancing the Research, and Mitigating the Effects: Workshop Summary. National Academies Press; 2004. www.ncbi.nlm.nih.gov/books/NBK83685/?report=reader. Accessed on Sept. 1, 2020.
2 Scholz CFP, Kilian M. Int J Syst Evol Microbiol. 2016;66(11):4422-4432.
3 Meisler DM et al. Am J Ophthalmol. 1986;102(6):733-739.
4 Clark WL et al. Ophthalmology. 1999;106(9):1665-1670.
5 Aldave, AJ et al. Ophthalmology. 1999;106(12):2395-2401.
6 Tetz MR et al. Arch Ophthalmol. 1987;105(10):1324-1325.
___________________________
Dr. Gelinas is a third-year ophthalmology resident, Dr. Palestine is professor of ophthalmology and chief of uveitis, and Dr. Pecen is a vitreoretinal surgeon and uveitis specialist and assistant professor of ophthalmology; all three are at the University of Colorado Sue Anschutz-Rodgers Eye Center in Aurora. Financial disclosures: None.