Download PDF
Five years of patient follow-up has confirmed that late endothelial cell loss occurs in mild to moderate open-angle glaucoma (OAG) patients implanted with the CyPass supraciliary microstent, combined with cataract surgery. But the remaining cells continued to keep the corneas clear, and the microstent reduced intraocular pressure (IOP) throughout the study.1,2
Alcon withdrew the CyPass from the market in August 2018, and the FDA later issued a class 1 recall. These steps were triggered by earlier monitoring results, which suggested that CyPass-treated eyes had accelerated cell loss at four and five years after surgery.
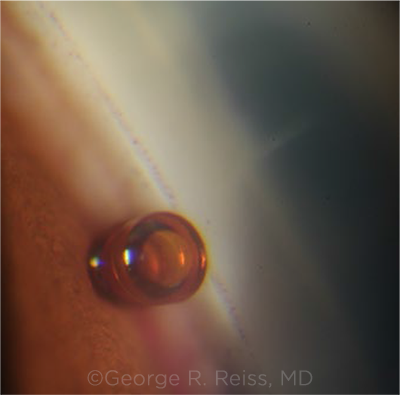 |
IN PLACE. A well-positioned CyPass in the supraciliary space with distal opening in front of the trabecular meshwork.
|
Recent findings. COMPASS XT investigators reported a mean decrease in endothelial cell density (ECD) of 20.4% in the CyPass eyes at five years after the combined surgery, compared to a mean 10.1% drop in the phacoemulsification-only control eyes.
This contrasted with the results at two years, when there was no difference in mean ECD between the two groups, said Jonathan H. Lass, MD, at Case Western Reserve University and University Hospitals Eye Institute in Cleveland.
“This is going to alert cornea and glaucoma specialists to be looking more closely at these patients over the long term. And then if they see signs of endothelial cell loss, such as the development of localized or diffuse striae and/or corneal edema, to do specular microscopy to evaluate the eyes further,” Dr. Lass said. “I would also refer clinicians to the ASCRS consensus statement regarding management.”3
Other five-year outcomes with the CyPass device were positive, said principal investigator George R. Reiss, MD, at Eye Physicians and Surgeons of Arizona, in Glendale and Scottsdale. The device was “well tolerated in the majority of patients,” he noted. “As compared with phacoemulsification alone, the group with CyPass had better pressure reduction and was more likely to be off medications.”
At five years, the mean reduction in IOP was slightly greater in the microstent cohort (8.4 mm Hg; 95% confidence interval [CI]: 7.8-8.9) than in the control group (8.0 mm Hg; 95% CI: 6.8-9.2), the investigators reported. (However, the study was not powered sufficiently to evaluate effectiveness with statistical significance.)
Adverse events were few, and there were no serious device-related adverse events. Three of the eyes had transient focal corneal edema in the region of the microstent at 33, 55, and 60 months, but no persistent corneal edema occurred in any eyes, the researchers reported.
Innate resilience? Previous studies of other causes of endothelial damage have shown that the endothelium is resilient, and this possibly explains why the corneas of patients who had received the CyPass remained healthy despite localized cell loss, Dr. Lass said.
“The issue is not the absolute endothelial cell density so much as it is the rapidity of the damage occurring. Because, even at low cell counts, the remaining cells—if given the chance—have the ability to adapt,” Dr. Lass said. “They get larger, change shape and size, increase the number of pump sites on their lateral margin, and migrate over to mitigate the damage. That’s probably what’s happening here, but further studies are needed.”
Looking ahead. CyPass was originally approved for mild to moderate glaucoma, but it might be more appropriate for use in more severe glaucoma cases in which surgical IOP reduction without bleb formation is desired, the COMPASS XT investigators suggested.
“Hopefully the device will make its way back into the market,” Dr. Reiss said. “Many glaucoma specialists miss this supraciliary stenting option.”
—Linda Roach
___________________________
1 Reiss G et al. Am J Ophthalmol. Published online Aug. 1, 2019.
2 Lass JH et al. Am J Ophthalmol. Published online Aug. 1, 2019.
3 Rhee D et al. Preliminary ASCRS CyPass Withdrawal Consensus Statement. https://ascrs.org/CyPass_Statement. Accessed on Sept. 18, 2019.
___________________________
Relevant financial disclosures—Dr. Lass: Alcon: S; Transcend Medical: S. Dr. Reiss: Aerie: L; Alcon: C,L,S; Allergan: C,S; Bausch Medical: L; Glaukos: L; Santen/InnFocus: C,S.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Chen National Institute on Aging: S; Quark Pharmaceuticals: C.
Dr. Drack Chakraborty Foundation: S; Canadian FFB: S; NIH: S; ProQr; S; Retrophin: S; Spark: S; Vision for Tomorrow: S.
Dr. Lass Alcon: S; Eversight Ohio: C; Transcend Medical: S.
Dr. Reiss Aerie: L; Alcon: C,L,S; Allergan: C,S; Bausch Medical: L; Glaukos: L; Haag-Streit: L; Santen/InnFocus: C,S.
Dr. Sauer Novartis: C; Tesseract: C.
Dr. Utz Retrophin: S; Springer Publishing: P.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review